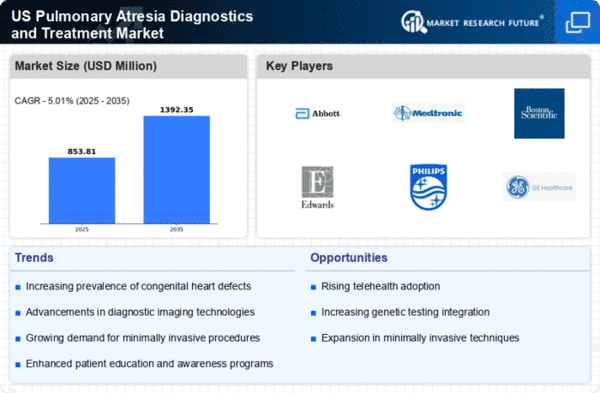
Rising Healthcare Expenditure
Rising healthcare expenditure in the US is a significant factor impacting the pulmonary atresia-diagnostics-treatment market. As healthcare spending continues to increase, there is a corresponding rise in investment in advanced medical technologies and treatment options. The US healthcare expenditure is projected to reach approximately $6 trillion by 2027, which may lead to enhanced funding for pediatric healthcare services, including those focused on congenital heart defects. This trend suggests that healthcare providers will have greater resources to allocate towards the diagnosis and treatment of pulmonary atresia, ultimately driving market growth. Increased funding may also facilitate research and development efforts, further expanding the range of available treatment options.
Growing Awareness and Education
The growing awareness and education surrounding congenital heart defects, particularly pulmonary atresia, are vital drivers for the pulmonary atresia-diagnostics-treatment market. Increased public and professional education initiatives have led to better recognition of symptoms and the importance of early diagnosis. Organizations dedicated to congenital heart disease advocacy are actively promoting awareness campaigns, which contribute to higher rates of diagnosis and treatment. This heightened awareness is expected to translate into increased demand for diagnostic services and treatment options, thereby propelling the growth of the pulmonary atresia-diagnostics-treatment market. As more healthcare providers become informed about the condition, the likelihood of timely interventions increases.
Government Initiatives and Funding
Government initiatives and funding aimed at improving healthcare access and outcomes significantly influence the pulmonary atresia-diagnostics-treatment market. Programs designed to support research and development in pediatric cardiology have led to increased investment in diagnostic and treatment technologies. For example, the National Institutes of Health (NIH) allocates substantial funding for research on congenital heart defects, which includes pulmonary atresia. This financial support fosters innovation and encourages collaboration between academic institutions and industry stakeholders. As a result, the pulmonary atresia-diagnostics-treatment market is likely to benefit from enhanced research capabilities and the development of new therapeutic options.
Technological Innovations in Medical Devices
Technological advancements in medical devices play a pivotal role in shaping the pulmonary atresia-diagnostics-treatment market. Innovations such as 3D imaging, minimally invasive surgical techniques, and advanced monitoring systems enhance the accuracy of diagnoses and the effectiveness of treatments. For instance, the integration of artificial intelligence in imaging technologies has shown promise in improving diagnostic precision. The market for medical devices related to pulmonary atresia is projected to grow, with estimates suggesting a compound annual growth rate (CAGR) of around 8% over the next five years. These innovations not only facilitate better patient management but also drive competition among manufacturers, leading to improved product offerings.
Increasing Incidence of Congenital Heart Defects
The rising incidence of congenital heart defects, including pulmonary atresia, is a critical driver for the pulmonary atresia-diagnostics-treatment market. Recent data indicates that congenital heart defects affect approximately 1 in 100 live births in the US, leading to a growing demand for effective diagnostic and treatment options. As awareness of these conditions increases, healthcare providers are more likely to seek advanced diagnostic tools and treatment modalities. This trend is further supported by the need for early detection and intervention, which can significantly improve patient outcomes. Consequently, the pulmonary atresia-diagnostics-treatment market is expected to expand as healthcare systems adapt to address the needs of this patient population.