Increased Healthcare Expenditure
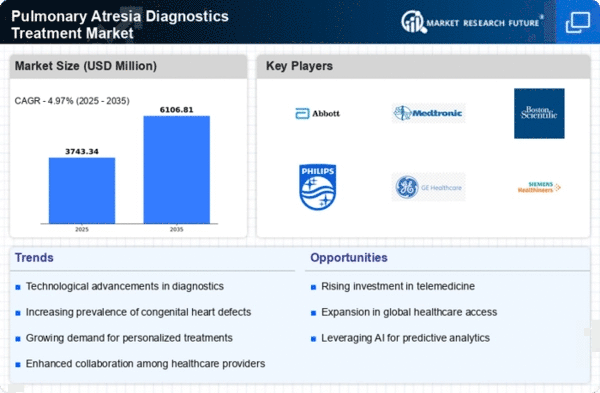
The rise in global healthcare expenditure is a crucial factor propelling the Global Pulmonary Atresia Diagnostics and Treatment Market Industry. Governments and private sectors are investing more in healthcare infrastructure and services, particularly in pediatric cardiology. This increased funding facilitates the development and availability of advanced diagnostic tools and treatment options for pulmonary atresia. As healthcare systems evolve, the focus on specialized care for congenital heart defects becomes more pronounced. This trend is likely to contribute to a compound annual growth rate (CAGR) of 6.0% from 2025 to 2035, indicating a robust market trajectory driven by enhanced healthcare investments.
Advancements in Diagnostic Technologies
Technological advancements in diagnostic imaging and genetic testing are significantly influencing the Global Pulmonary Atresia Diagnostics and Treatment Market Industry. Innovations such as 3D echocardiography and MRI have improved the accuracy of diagnosing pulmonary atresia, allowing for earlier and more precise interventions. These advancements not only enhance patient outcomes but also drive market growth as healthcare providers increasingly adopt these technologies. The integration of artificial intelligence in diagnostic processes further streamlines patient assessment and management. As a result, the market is expected to expand, with a projected value of 7.44 USD Billion by 2035, reflecting the impact of these technological improvements.
Growing Awareness and Screening Programs
The growing awareness of congenital heart diseases and the implementation of screening programs are vital drivers of the Global Pulmonary Atresia Diagnostics and Treatment Market Industry. Public health initiatives aimed at educating parents and healthcare providers about the signs and symptoms of pulmonary atresia have led to earlier diagnoses and interventions. Screening programs, particularly in newborns, have become more prevalent, resulting in timely treatment and improved patient outcomes. This increased awareness is expected to sustain market growth, with projections indicating a market value of 3.92 USD Billion in 2024. The proactive approach to identifying and managing pulmonary atresia is likely to enhance overall healthcare delivery.
Collaborations and Partnerships in Research
Collaborations between healthcare institutions, research organizations, and pharmaceutical companies are fostering innovation in the Global Pulmonary Atresia Diagnostics and Treatment Market Industry. These partnerships facilitate the development of novel therapies and diagnostic tools tailored to the needs of patients with pulmonary atresia. By pooling resources and expertise, stakeholders can accelerate research and bring new solutions to market more efficiently. Such collaborations are crucial for addressing the complexities of congenital heart defects and ensuring that advancements reach the clinical setting. This collaborative environment is expected to contribute to sustained market growth, aligning with the projected CAGR of 6.0% from 2025 to 2035.
Rising Prevalence of Congenital Heart Defects
The increasing incidence of congenital heart defects, including pulmonary atresia, is a primary driver of the Global Pulmonary Atresia Diagnostics and Treatment Market Industry. According to health statistics, congenital heart defects affect approximately 1 in 100 live births globally. This rising prevalence necessitates enhanced diagnostic and treatment options, contributing to the market's growth. As awareness and screening programs improve, more cases are identified, leading to a projected market value of 3.92 USD Billion in 2024. This trend indicates a growing demand for innovative diagnostic tools and treatment methodologies to address the needs of affected infants.