Market Trends
Key Emerging Trends in the Uterine Fibroid Treatment Device Market
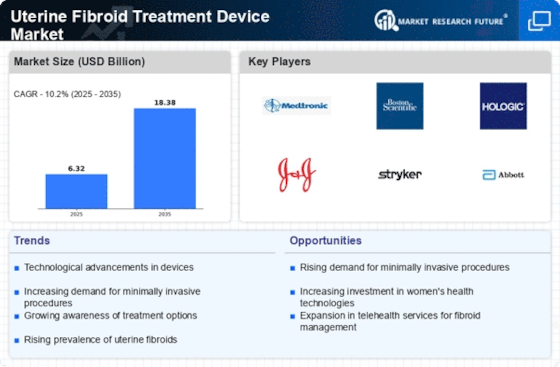
Recent progressions in women’s health, non-invasive methods, and the need for effective treatment alternatives are the primary ideologies behind key trends in the Uterine Fibroid Treatment Device Market. As a result, there has been an increased demand for novel devices that can be utilized to treat this illness. Noting that over the years, several important factors have defined the Uterine Fibroid Treatment Device Market including patient-focused approach, technology innovation and move from traditional surgical interventions.
A major trend that is emerging in the Uterine Fibroid Treatment Device Market is the growing uptake of minimally invasive techniques for fibroid treatment. Some of these procedures include laparoscopic or hysteroscopic surgeries and non-surgical treatments such as uterine artery embolization (UAE) and focused ultrasound therapy. In line with general healthcare trends towards less invasive techniques with shorter recovery periods, these techniques offer reduced hospital stays, lower complications rates and higher satisfaction levels among patients compared to traditional open surgery.
Technological advances in fibroid treatment devices are also spurring innovation within the market. Advanced imaging technologies such as magnetic resonance imaging (MRI) guidance for focused ultrasound therapy improve precision and accuracy of treating fibroids while new radiofrequency ablation (RFA) and microwave ablation technologies add more choices to enable medical professionals effectively targeting and shrinking uterine fibroids. Application of advanced technologies sets stage for numerous shots at curing diseases with respect to individual needs thereby expanding on options when it comes to curative measures.
Additionally, there is a shift towards outpatient-based practices in managing fibroids using specialized devices within this market segment. Outpatient-based surgery offers high accessibility due to decreased time spent by patients at hospitals among other advantages associated with fewer infections which results from use of sterile operating environments. Consequently, many women can go home soon after undergoing their treatments or avoid hospital admissions completely thus giving them a chance to recuperate quickly and get back to their daily lives. This is in line with growing emphasis on patient-centered care and increased access to healthcare.
Similarly, there is a growing preference for uterine-preserving treatment options within the market. In light of this development, women with fibroids are increasingly concerned about preserving fertility and focus mainly on treatment techniques that can facilitate this while at the same time keeping the womb open for possible future pregnancies. Some minimally invasive procedures such as hysteroscopic myomectomy and fertility-sparing fibroid embolization cater to such individuals’ demands thereby indicating how individualized care is important within the Uterine Fibroid Treatment Device Market.
In addition, there has been a move towards increasing patient awareness in the Uterine Fibroid Treatment Device Market. As more women learn about uterine fibroids and available treatment options, they want to be involved in decision making concerning their health. Early detection through heightened awareness leads to timely treatment hence better outcomes. Patient education therefore falls into place as an effort by healthcare providers to allow people manage their health issues and choose wisely on what they need done.
Medical device manufacturers, health care institutions as well as research organizations are the forces behind research and development efforts in Uterine Fibroid Treatment Device Market. These partnerships enable knowledge exchange, clinical insights, and technology expertise that led to invention of new treatment devices and methods. The cooperation between industry players and medical practitioners is promoting innovations in fibroid treatments hence promising better patient outcomes.
Despite these positive trends, a number of challenges remain in the Uterine Fibroid Treatment Device Market such as long-term efficacy data requirements, insurance coverage concerns, and disparities in access to advanced treatments. Longitudinal studies to determine emerging therapies’ effectiveness; improved insurance coverage advocacy; and awareness-raising campaigns to extend the scope of patients are some of strategies used toward these ends.
















Leave a Comment