Market Analysis
In-depth Analysis of Vascular Embolization Market Industry Landscape
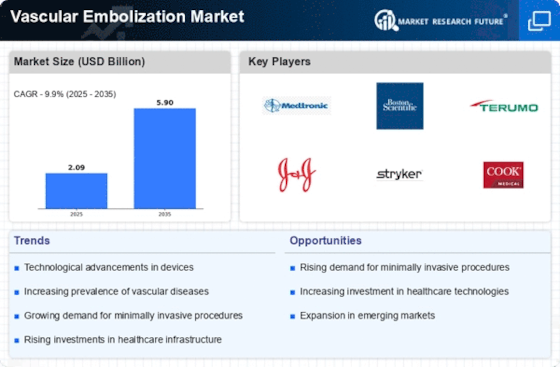
The global vascular embolization market is set to reach US$ 4.0 BN by 2032, at a 9.90% CAGR between years 2023-2032. The vascular embolization market works on an environment which is highly dynamic in nature, where various elements are propelling the requirement of newer innovations and devices that fall under this category. An important factor is growing incidence of vascular disorders and diseases as aneurisms or arteriovenous malformations. As the prevalence of these conditions increases around the world, so does demand to respond effectively with embolization solutions that shape market dynamics in response to this growing need. The market dynamics are greatly influenced by the technological advances in embolization devices. The constant innovations in materials, design, and delivery systems further lead to better embolization techniques with increasing precision and minimally invasive nature. The market competitively responds to such technological advances by providing a series of high-quality devices which help improve patient safety, efficiency, and versatility in vascular embolization procedures. Regulatory issues and compliance standards are among the major drivers of market trends. Thus, patient safety and efficacy of new embolization devices are verified during the approval and clearance processes under highly regulated conditions. Strategies of market players are influenced by compliance with regulatory requirements, which also determines product development, clinical trials, and entry into the markets. Embolization devices gain credibility and trustworthiness in the healthcare environment with a well-developed regulatory framework. Market dynamics include economic factors such as healthcare spending and cost-effectiveness. The goal of healthcare providers in finding an embolization solution is to strike a balance between cost and quality. Depending on the cost-effectiveness of embolization procedures and devices, adoption of new technologies is dictated determining market trends while healthcare institutions seek to reduce wastages. The dynamics of the vascular embolization market are influenced by globalisation and growth in markets. Companies are increasing market penetration through strategic alliances, collaborations as well as distribution agreements which provide them with access to new geographic regions. This globalization creates a more interdependent market wherein the manufacturers customize their products to suit those need which vary in healthcare providers and patients all over the world. Aging population and the resulted increase in age-related vascular conditions have a great influence on market dynamics. As the population continues to get older, some of these conditions such as cerebral aneurysms and peripheral artery disease become prevalent. The market addresses this demographic trend by emphasizing the development of embolization devices suitable for older individuals, focusing on issues and aspects related to aging vascular structures.















Leave a Comment