Market Share
Venous Blood Collection Devices Market Share Analysis
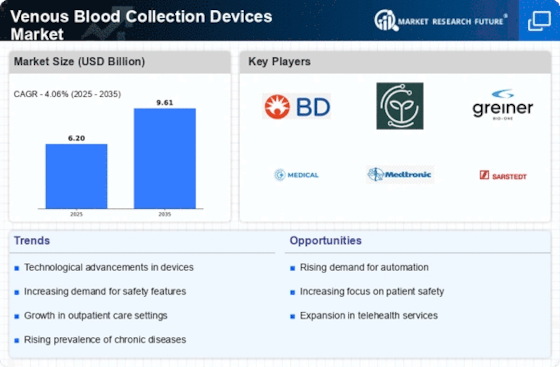
Companies must investigate the competition to determine their Venous Blood Collection Devices market share. Companies must understand market size, trends, competition, and healthcare workers' preferences to build targeted strategies.
Businesses that desire a substantial market share regularly develop new items. Advanced arterial blood collection equipment with improved safety, easier usage, and sample compatibility stand out in a competitive market and attract healthcare personnel seeking cutting-edge solutions. Successful companies provide bespoke solutions since venous blood collection may be employed in various medical sectors. By creating devices that function in hospitals, clinics, and laboratories, they become adaptable service providers that can suit many demands. Working with hospitals and labs is crucial to market position. Companies develop smart agreements to ensure their venous blood collection devices function with other systems. These agreements allow consumers to trial items, offer real-world feedback, and get healthcare community attention. Full training and education programs are essential for market share expansion. Companies train medical staff to use their devices appropriately, focusing on ways that make blood collection fast and painless for patients and provide reliable sample findings. Users who utilize electronics properly succeed and have a good reputation. Foreign expansion is a common strategy for companies seeking market share. Because various places have distinct healthcare systems and restrictions, they tailor their products and marketing to each area. Going worldwide increases their consumer base and makes them global leaders.
Safety and compliance must constantly be prioritized in arterial blood collection equipment. Healthcare personnel trust companies that invest in needle stick and infection prevention measures, reducing their concerns about procedure risks. Supply chain stability is crucial for market share positioning. Companies that satisfy demand, minimize product gaps, and deliver on time strengthen relationships with healthcare personnel. A well-run supply chain improves customer satisfaction and market image. Venous blood collection equipment companies employ digital technology because it improves healthcare. Reading barcodes, connecting to LIS, and digitally tracking material data are examples. This merger improves efficiency, reduces errors, and positions the organization ahead in digital health. Effective marketing and branding are essential for market share growth. Companies tout the advantages of their venous blood-collecting equipment in various ways. A company may distinguish out by emphasizing reliability, fresh ideas, and user-friendly features.


















Leave a Comment