Summary Overview
Gene Therapy Trails Market Overview
The global gene therapy trials market is expanding rapidly, driven by advances in science and rising demand for breakthrough medicines in a variety of therapeutic areas including oncology, rare genetic disorders, and neurology. This market encompasses a wide range of gene therapy techniques, including viral vector-based, non-viral vector, and CRISPR/Cas9 gene editing platforms. Our research includes a detailed examination of clinical trial trends, with a focus on cost-cutting tactics and the use of cutting-edge technologies to improve trial efficiency and therapeutic effects.
Key potential problems in gene therapy studies include controlling high clinical development costs, assuring treatment scalability, dealing with regulatory complications, and incorporating novel gene therapy procedures into established medical frameworks. Digital technologies and strategic sourcing are vital for refining trial designs, increasing patient recruitment, and assuring long-term viability in the competitive gene therapy landscape. As global demand for personalized medicine and targeted treatments continues to rise, companies are leveraging advanced analytics and market intelligence to streamline trial processes, mitigate risks, and enhance treatment effectiveness.
-
Market Size: The global Gene Therapy Trails market is projected to reach USD 128.8 billion by 2035, growing at a CAGR of approximately 18.2% from 2025 to 2035

- Growth Rate: 18.2%
-
Sector Contributions: Growth in the market is driven by:
-
Manufacturing and Supply Chain Optimization in Gene Therapy: There is an increasing demand for real-time data and process integration to improve trial logistics, patient enrolment, and material management.
-
Increased Clinical Trial and Patient Recruitment: Implemented advanced systems for better patient recruitment, site management, and monitoring. These technologies improve the management of trial data, patient relations, and regulatory reporting.
-
Technological Transformation in Gene Therapy Trials: Advances in AI and machine learning improve clinical trial designs, predict patient outcomes, and automate data processing.
-
Innovations in Clinical Trial Design: Modular clinical trial solutions allow organizations to pick and integrate the required components the specific needs of their gene therapy development, reducing costs, complexity, and trial duration.
-
Investment Initiatives: Significant funds are being invested in gene therapy trial infrastructure, notably in gene editing technologies and next-generation treatments. Given the high expenses of research and development, these investments are critical for promoting innovation.
-
Regional Perspectives on Gene Therapy Trials: North America and Asia Pacific continue to dominate the gene therapy trials market, thanks to strong healthcare infrastructure, superior digital capabilities, and the increased usage of cloud-based solutions for handling data, patient recruiting, and trial monitoring.
Key Trends and Sustainability Outlook
-
Cloud Integration: Data access, scalability, and cost efficiency have all improved significantly as cloud technologies become more widely used in clinical trials. Cloud-based solutions also allow for improved communication among global research teams, increasing the speed and precision of gene therapy studies.
-
Advanced features: The integration of AI, machine learning, and data analytics allows researchers to gain predictive insights, optimize clinical trial designs, and improve therapy tailoring.
-
Focus on Sustainability: To maintain long-term health, gene therapy development should prioritize sustainable trial designs and operations, as well as efficient resource tracking goals and compliance with environmental standards.
-
Customization Trends: Customization ensures that studies are tailored to specific therapeutic areas and patient demands, which is crucial for increasing the efficacy of gene therapies.
-
Data-Driven Insights: Using sophisticated analytics in gene therapy trials improves monitoring, patient selection, and decision-making. The capacity to predict trial outcomes and enhance patient treatments using data-driven insights is changing how gene therapies are created.
Growth Drivers:
-
Digital Transformation: The healthcare industry is leveraging digital technologies to improve speed and precision in gene therapy studies.
-
Demand For Process Automation: Automated methods are increasingly used in gene therapy trials to decrease errors, speed up data processing, and ensure accuracy.
-
Scalability Requirements: As gene therapy studies develop globally, there is a high demand for scalable clinical trial models. These models ensure that trials may be altered when new technologies and insights emerge, allowing them to evolve alongside advances in gene therapy.
-
Regulatory compliance: Given the complexities of gene treatments, adhering to regulatory criteria is important to the success of trials. Advanced systems ensure regulatory compliance by automating paperwork, reporting, and data management.
-
Globalization: Global collaborations and studies are critical for increasing access to cutting-edge gene treatments, assuring compliance with international standards, and serving various patient groups.
Overview of Market Intelligence Services for the Gene Therapy Trails Market:
Recent evaluations have found numerous important problems in the gene therapy trials market, including high research and development costs, regulatory barriers, and the complexities of patient recruiting. Market intelligence studies provide significant insights into chances to improve trial efficiency, assisting organizations in identifying cost-saving methods, streamlining clinical processes, and increasing overall success in gene therapy development. These insights are also critical for meeting regulatory requirements, enhancing trial quality, and successfully managing costs throughout the therapeutic development lifecycle.
Procurement Intelligence for Gene Therapy Trails: Category Management and Strategic Sourcing
To preserve a competitive advantage in the gene therapy trial market, organizations are streamlining procurement procedures through trial design analysis and vendor performance tracking. Effective category management and strategic sourcing are crucial for lowering costs connected with clinical trial operations and assuring a consistent supply of high-quality gene therapy solutions. By leveraging actionable market intelligence, businesses can refine their trial strategies, negotiate favourable terms with service providers, and ensure the successful execution of gene therapy trials.
Pricing Outlook for Gene Therapy Trails: Spend Analysis
The pricing prognosis for gene therapy trials is projected to remain moderately dynamic, impacted by several critical factors. Advancements in gene-editing technology, increased desire for individualized medicines, regulatory difficulties, and geographic variances in trial costs are all major factors. Furthermore, the expanding use of AI and machine learning in clinical trials, together with increased requirements for patient data security and compliance, is expected to drive up overall gene therapy trial costs.
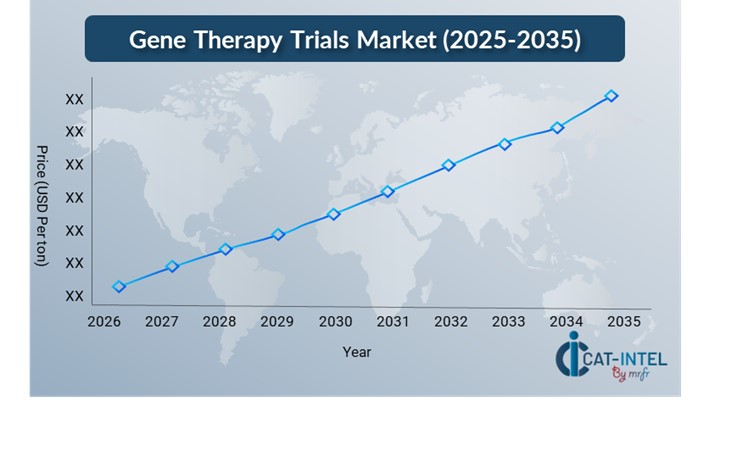
Graph shows general upward trend pricing for Gene Therapy Trials and growing demand. However, there may be fluctuations influenced by economic conditions, technological advancements, and competitive dynamic
Efforts to optimize clinical trial processes, improve management of suppliers, and implement flexible trial designs are crucial for cost containment. Using digital technologies for market monitoring, anticipating trial costs, and harnessing analytics to optimize patient recruitment can increase total cost efficiency.
Collaborating with experienced clinical trial service providers to negotiate multi-year contracts for services and exploring cost-effective pricing models will be essential for managing trial-related expenses. Despite these hurdles, focusing on scalability, ensuring rigorous regulatory compliance, and implementing advanced data management systems will be critical to assuring cost-effectiveness and excellent trial outcomes.
Cost Breakdown for Gene Therapy Trails: Total Cost of Ownership (TCO) and Cost-Saving Opportunities
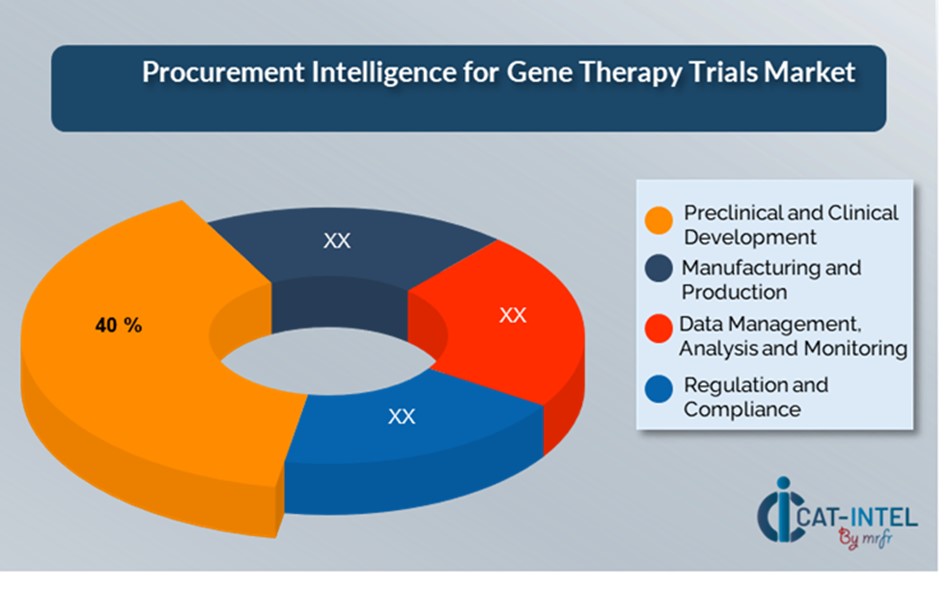
- Preclinical and Clinical Development: (40%)
-
Description: This phase includes the initial research, laboratory testing, animal studies, and clinical trials (Phase I, II, and III). It comprises all activities required to assess the safety and efficacy of the gene therapy product before it is approved for widespread usage.
-
Trend: There is a growing trend towards accelerated clinical trial timelines employing adaptive trial designs and using technology like artificial intelligence (AI) and patient recruiting platforms, which can assist streamline the process, cut costs, and increase the successful outcomes
- Manufacturing and Production: (XX%)
- Regulation and Compliance: (XX%)
- Data Management, Analysis, and Monitoring: (XX%)
Cost-Saving Opportunities: Negotiation Levers and Purchasing Negotiation Strategies
In the gene treatment trials industry, streamlining procurement processes and using strategic bargaining strategies can result in significant cost reductions and increased trial efficiency. Long-term agreements with clinical trial service providers, particularly those that offer innovative technologies like gene-editing platforms and AI-powered patient monitoring systems, can lead to more attractive pricing structures and conditions. This could include volume-based discounts, packaged service packages, and trial designs that are suited to specific therapeutic areas. Using subscription-based models and multi-year contracts for services like patient recruiting, regulatory support, and data management can help achieve cheaper prices and protect against cost fluctuations.
Partnering with innovative providers who prioritize scalability and cutting-edge technologies enables the integration of advanced analytics, real-time monitoring, and flexible trial frameworks, resulting in lower operational costs and faster time-to-market for new medicines. Implementing digital procurement technologies, such as trial management platforms and data analytics software, improves transparency, optimizes resource allocation, and reduces inefficiencies in clinical trial execution. Furthermore, diversifying vendor options and implementing a multi-vendor approach decreases reliance on a single service provider, lowering the risk of operational delays or regulatory noncompliance while increasing negotiation leverage for better terms.

Supply and Demand Overview for Gene Therapy Trails: Demand-Supply Dynamics and Buyer Intelligence for Effective Supplier Relationship Management (SRM)
The gene therapy trials market is steadily expanding, driven by advances in gene-editing technology, rising desire for customized treatment, and regulatory developments in various regions. Biotechnology advancements, the requirement for regulatory compliance, and global economic situations all have an impact on supply and demand dynamics.
Demand Factors:
-
Advances in Gene Therapy Technologies: The growing need for focused and individualized treatments is driving demand for gene therapies in fields such as oncology, uncommon diseases, and genetic disorders.
-
Regulatory Support and Flexibility: Increased expenditure on regulatory frameworks for gene treatments, combined with advances in approval processes, is driving demand for clinical trials and accelerating market access.
-
Cost and Efficiency Considerations: There is an increasing demand for trials that balance efficiency and innovation, pushing for enhanced clinical trial designs and optimized patient recruitment strategies.
-
Integrate with Digital Health Tools: The growing incorporation of digital health technology, such as wearables and mobile apps, into gene therapy studies improves patient data collecting and real-time monitoring, resulting in higher trial efficiency.
Supply Factors:
-
Technological Advancements: Innovations in CRISPR, gene editing, and AI-powered data analysis are greatly improving gene therapy development and increasing market competitiveness among suppliers.
-
Expanding Vendor Ecosystem: With an expanding number of biotech firms, clinical research organizations (CROs), and technology suppliers specializing in gene therapy studies, trial sponsors have a choice of options to choose from.
-
Global Economic Factors: Economic variables such as labour costs, currency volatility, and regional biotech investment rates affect gene therapy trial price and development timetables.
-
Scalability and Customization: As technology advances and patient-centric trial designs improve, gene therapy trials become more scalable and customizable to address the demands of specific patient populations.
Regional Demand-Supply Outlook: Gene Therapy Trails
The Image shows growing demand for Gene Therapy Trials in both North America and Asia Pacific, with potential price increases and increased Competition.
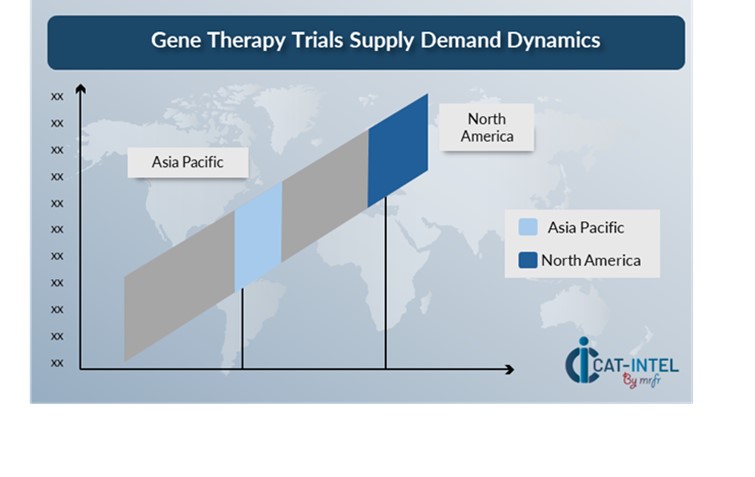
North America: Dominance in the Gene Therapy Trials Market
North America, particularly the United States, is a dominant force in the global Gene Therapy Trials market due to several key factors:
-
Strong Regulatory Support: North America, particularly the United States, has a well-established regulatory framework (FDA) that provides clear rules and expedited approval processes, such as the Orphan Drug and Breakthrough Therapy designations, which promote gene therapy innovation.
-
High R&D Investment: North American companies, notably biotech and pharmaceutical heavyweights, engage extensively in gene therapy research and development, which is supported by public and private funding, making it a key role in the advancement of gene treatments.
-
Advanced Healthcare Infrastructure: The region has cutting-edge healthcare facilities, cutting-edge laboratories, and highly trained clinical researchers, making it an excellent location for gene therapy studies.
-
Access to big Patient Pools: North America's diversified and big patient population provides ample opportunity for patient recruitment in gene therapy trials, facilitating the collection of critical clinical data across various therapeutic areas.
-
Academia-Industry Collaboration: North America is home to multiple world-class universities and research institutes that work closely with the biotech and pharmaceutical industries to stimulate innovation and accelerate the development of gene treatments.
North America Remains a key hub Gene Therapy Trials Price Drivers Innovation and Growth.
Supplier Landscape: Supplier Negotiations and Strategies
The gene therapy trials market has a highly competitive and diverse supplier landscape, with global industry leaders and specialized regional players influencing the dynamics. These suppliers have a significant impact on trial designs, patient recruitment, regulatory compliance, and the incorporation of innovative technologies. The market is controlled by well-known biotechnology companies and contract research organizations (CROs), who offer comprehensive services for large-scale studies. Smaller, niche suppliers specialize on certain therapeutic areas or provide unique solutions such as patient management systems, gene editing technology, and advanced analytics.
The gene therapy trials ecosystem in major biotechnology regions includes both worldwide vendors with broad service offerings and local innovators who address the regulatory, clinical, and technological requirements of specialty therapeutic areas. As the industry prioritizes personalized medicine and rapid clinical development, gene therapy suppliers are expanding their technological capabilities, incorporating AI-driven data analytics, and providing more flexible trial designs to meet the changing needs of sponsors and researchers. This developing ecosystem is critical for expediting gene therapy trial processes, assuring compliance, and shortening the time-to-market for innovative treatments.
Key Suppliers in the Gene Therapy Trials market include:
- Gilead Sciences
- Novartis AG 2., Inc.
- Spark Therapeutics
- Bluebird Bio, Inc.
- Bayer AG
- Sarepta Therapeutics, Inc
- Biogen Inc.
- AbbVie Inc
- CRISPR Therapeutics
- Viatris Inc.
Key Developments Procurement Category Significant Development:
Significant Development |
Description |
Market Growth |
The gene therapy trials market is expanding rapidly, owing to advances in gene-editing technology, increased investments in personalized medicine, and stronger regulatory backing. |
Cloud Adoption |
Cloud solutions allow for global cooperation, real-time data sharing, and clinical data management, which is critical for decentralized trial designs and patient recruitment. |
Product Innovation |
Gene therapy businesses are concentrating on advances in genetic engineering technologies such as CRISPR and viral vector creation. These breakthroughs improve the precision of gene therapies, and AI-powered analytics are being used in trials to optimize patient selection. |
Technological Advancements |
New technologies like machine learning, IoT devices for patient monitoring, and robotic process automation (RPA) are improving gene therapy experiments. These advances improve patient recruiting and real-time data analysis, , and automating administrative tasks, which accelerates trial timelines and improves trial accuracy. |
Global Trade Dynamics |
Changes in global regulatory frameworks, compliance requirements, and economic policies all have an impact on gene therapy trial uptake. Multinational corporations handling complicated gene therapy trials must traverse varied regulations in multiple markets, necessitating standardized yet adaptive trial designs. |
Customization Trends |
There is a growing demand for customized gene therapy trial solutions that are suited to certain clinical areas. This involves tailored patient monitoring, specialized treatment procedures, and the incorporation of third-party technologies, such as enhanced diagnostic tools or digital health platforms, to improve trial outcomes. |
|
Gene Therapy Trails Attribute/Metric |
Details |
Market Sizing |
The global Gene Therapy Trails market is projected to reach USD 128.8 billion by 2035, growing at a CAGR of approximately 18.2% from 2025 to 2035. |
Gene Therapy Trails Technology Adoption Rate |
The adoption rate of gene therapy trial technologies is gradually rising about 60%, with a noticeable shift toward cloud-based platforms and digital health solutions to improve patient recruitment, data collection, and real-time monitoring for trial efficiency.
|
Top Gene Therapy Trails Industry Strategies for 2025 |
Key strategies in gene therapy trials include integrating AI and machine learning for predictive patient results and trial efficiency, optimizing registration of patients with digital health tools, focusing on data security to meet stringent regulatory requirements, and improving trial accessibility with mobile platforms for remote patient monitoring. |
Gene Therapy Trails Process Automation |
Approximately 50% of gene therapy trials use automation in areas such as patient monitoring, data gathering, and regulatory reporting, considerably boosting operational efficiency and reducing the manual effort.
|
Gene Therapy Trails Process Challenges |
Key problems in the gene therapy trial business include high research and development costs, regulatory hurdles, issues with patient recruitment, data integration across many platforms, and the complexity of gene delivery methods.
|
Key Suppliers |
Leading gene therapy suppliers include Gilead Sciences, Novartis AG 2., Inc. and Spark Therapeutics, with an emphasis on cutting-edge technologies such as CRISPR/Cas9 gene editing and viral vector development.
|
Key Regions Covered |
Prominent regions for gene therapy trials include North America, Europe, and Asia-Pacific, with significant growth driven by advancements in biotechnology, increased healthcare investments, and an increasing number of clinical trials in oncology, genetic disorders, and rare diseases.
|
Market Drivers and Trends |
The increasing demand for personalized medicine, advancements in gene-editing technologies, a rise in genetic disorders and rare diseases, as well as a stronger emphasis on real-time data analytics and remote monitoring for trials, are all driving growth.
|










