-
FACTOR ANALYSIS
-
\r\n\r\n\r\nValue chain Analysis
-
\r\n\r\n\r\nPorter's
-
Five Forces Analysis
-
\r\n\r\n\r\nBargaining Power of Suppliers
-
\r\n\r\n\r\nBargaining
-
Power of Buyers
-
\r\n\r\n\r\nThreat of New Entrants
-
\r\n\r\n\r\nThreat
-
of Substitutes
-
\r\n\r\n\r\nIntensity of Rivalry
-
\r\n\r\n\r\n\r\n\r\nCOVID-19
-
Impact Analysis
-
\r\n\r\n\r\nMarket Impact Analysis
-
\r\n\r\n\r\nRegional
-
Impact
-
\r\n\r\n\r\nOpportunity and Threat Analysis
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\nHIV
-
Diagnostics Market, BY Test Type (USD Billion)
-
\r\n\r\n\r\nAntibody
-
Tests
-
\r\n\r\n\r\nAntigen Tests
-
\r\n\r\n\r\nNucleic
-
Acid Tests
-
\r\n\r\n\r\n\r\n\r\nHIV Diagnostics
-
Market, BY End User (USD Billion)
-
\r\n\r\n\r\nHospitals
-
\r\n\r\n\r\nDiagnostic
-
Laboratories
-
\r\n\r\n\r\nHome Care Settings
-
\r\n\r\n\r\n\r\n\r\nHIV
-
Diagnostics Market, BY Product Type (USD Billion)
-
\r\n\r\n\r\nTests
-
Kits
-
\r\n\r\n\r\nInstruments
-
\r\n\r\n\r\nSoftware
-
\r\n\r\n\r\n\r\n\r\nHIV
-
Diagnostics Market, BY Technology (USD Billion)
-
\r\n\r\n\r\nElisa
-
\r\n\r\n\r\nRapid
-
Testing
-
\r\n\r\n\r\nPolymerase Chain Reaction
-
\r\n\r\n\r\n\r\n\r\nHIV
-
Diagnostics Market, BY Regional (USD Billion)
-
\r\n\r\n\r\nNorth
-
America
-
\r\n\r\n\r\nUS
-
\r\n\r\n\r\nCanada
-
\r\n\r\n\r\n\r\n\r\nEurope
-
\r\n\r\n\r\nGermany
-
\r\n\r\n\r\nUK
-
\r\n\r\n\r\nFrance
-
\r\n\r\n\r\nRussia
-
\r\n\r\n\r\nItaly
-
\r\n\r\n\r\nSpain
-
\r\n\r\n\r\nRest
-
of Europe
-
\r\n\r\n\r\n\r\n\r\nAPAC
-
\r\n\r\n\r\nChina
-
\r\n\r\n\r\nIndia
-
\r\n\r\n\r\nJapan
-
\r\n\r\n\r\nSouth
-
Korea
-
\r\n\r\n\r\nMalaysia
-
\r\n\r\n\r\nThailand
-
\r\n\r\n\r\nIndonesia
-
\r\n\r\n\r\nRest
-
of APAC
-
\r\n\r\n\r\n\r\n\r\nSouth America
-
\r\n\r\n\r\nBrazil
-
\r\n\r\n\r\nMexico
-
\r\n\r\n\r\nArgentina
-
\r\n\r\n\r\nRest
-
of South America
-
\r\n\r\n\r\n\r\n\r\nMEA
-
\r\n\r\n\r\nGCC
-
Countries
-
\r\n\r\n\r\nSouth Africa
-
\r\n\r\n\r\nRest
-
of MEA
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\n\r\nCompetitive
-
Landscape
-
\r\n\r\n\r\nOverview
-
\r\n\r\n\r\nCompetitive
-
Analysis
-
\r\n\r\n\r\nMarket share Analysis
-
\r\n\r\n\r\nMajor
-
Growth Strategy in the HIV Diagnostics Market
-
\r\n\r\n\r\nCompetitive
-
Benchmarking
-
\r\n\r\n\r\nLeading Players in Terms of Number of Developments
-
in the HIV Diagnostics Market
-
\r\n\r\n\r\nKey developments and growth
-
strategies
-
\r\n\r\n\r\nNew Product Launch/Service Deployment
-
\r\n\r\n\r\nMerger
-
& Acquisitions
-
\r\n\r\n\r\nJoint Ventures
-
\r\n\r\n\r\n\r\n\r\nMajor
-
Players Financial Matrix
-
\r\n\r\n\r\nSales and Operating Income
-
\r\n\r\n\r\nMajor
-
Players R&D Expenditure. 2023
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\nCompany
-
Profiles
-
\r\n\r\n\r\nAbbott Laboratories
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nThermo Fisher Scientific
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nBecton Dickinson and Company
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nHologic Inc
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nMeridian Bioscience
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nGrifols
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nGenMark Diagnostics
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nDanaher Corporation
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nRoche Holding AG
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nQuidel Corporation
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nBioRad Laboratories
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nPerkinElmer
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nCepheid
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nOrtho Clinical Diagnostics
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\nSiemens Healthineers
-
\r\n\r\n\r\nFinancial
-
Overview
-
\r\n\r\n\r\nProducts Offered
-
\r\n\r\n\r\nKey
-
Developments
-
\r\n\r\n\r\nSWOT Analysis
-
\r\n\r\n\r\nKey
-
Strategies
-
\r\n\r\n\r\n\r\n\r\n\r\n\r\nAppendix
-
\r\n\r\n\r\nReferences
-
\r\n\r\n\r\nRelated
-
Reports
-
\r\n\r\n\r\n\r\n\r\nLIST Of tables
-
\r\n\r\n\r\nLIST
-
OF ASSUMPTIONS
-
\r\n\r\n\r\nNorth America HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nNorth
-
America HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nNorth America HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nNorth
-
America HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nNorth America HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUS
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nUS HIV Diagnostics Market SIZE ESTIMATES &
-
FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUS HIV
-
Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nUS HIV Diagnostics Market SIZE ESTIMATES &
-
FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUS
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nCanada HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nCanada
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nCanada HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nCanada
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nCanada HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nEurope
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nEurope HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nEurope
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nEurope HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nEurope
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nGermany HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGermany
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nGermany HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGermany
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nGermany HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUK
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nUK HIV Diagnostics Market SIZE ESTIMATES &
-
FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUK HIV
-
Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nUK HIV Diagnostics Market SIZE ESTIMATES &
-
FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nUK
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nFrance HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nFrance
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nFrance HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nFrance
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nFrance HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRussia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nRussia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRussia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRussia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRussia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nItaly HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nItaly
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nItaly HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nItaly
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nItaly HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSpain
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nSpain HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSpain
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSpain HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSpain
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nRest of Europe HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of Europe HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of Europe HIV Diagnostics Market
-
SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of Europe HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of Europe HIV Diagnostics Market
-
SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nAPAC
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nAPAC HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nAPAC
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nAPAC HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nAPAC
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nChina HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nChina
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nChina HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nChina
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nChina HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nIndia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nIndia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nJapan HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nJapan
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nJapan HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nJapan
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nJapan HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Korea HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth Korea HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Korea HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth Korea HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Korea HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nMalaysia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMalaysia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMalaysia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMalaysia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMalaysia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nThailand
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nThailand HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nThailand
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nThailand HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nThailand
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nIndonesia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndonesia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nIndonesia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nIndonesia
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nIndonesia HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of APAC HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of APAC HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of APAC HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of APAC HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of APAC HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth America HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
America HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth America HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
America HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth America HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nBrazil
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nBrazil HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nBrazil
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nBrazil HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nBrazil
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMexico HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMexico
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMexico HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMexico
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMexico HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nArgentina
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nArgentina HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nArgentina
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nArgentina HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nArgentina
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nRest of South America HIV Diagnostics Market
-
SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of South America HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER,
-
\r\n\r\n\r\nRest of South America HIV Diagnostics
-
Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of South America HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY,
-
\r\n\r\n\r\nRest of South America HIV Diagnostics
-
Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMEA
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMEA HIV Diagnostics Market SIZE ESTIMATES &
-
FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMEA HIV
-
Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nMEA HIV Diagnostics Market SIZE ESTIMATES &
-
FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nMEA
-
HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD
-
Billions)
-
\r\n\r\n\r\nGCC Countries HIV Diagnostics Market SIZE ESTIMATES
-
& FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGCC
-
Countries HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nGCC Countries HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nGCC
-
Countries HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nGCC Countries HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Africa HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth Africa HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY END USER, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Africa HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nSouth Africa HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nSouth
-
Africa HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY REGIONAL, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of MEA HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY TEST TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of MEA HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY END USER, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of MEA HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY PRODUCT TYPE, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nRest
-
of MEA HIV Diagnostics Market SIZE ESTIMATES & FORECAST, BY TECHNOLOGY, 2019-2035
-
(USD Billions)
-
\r\n\r\n\r\nRest of MEA HIV Diagnostics Market SIZE
-
ESTIMATES & FORECAST, BY REGIONAL, 2019-2035 (USD Billions)
-
\r\n\r\n\r\nPRODUCT
-
LAUNCH/PRODUCT DEVELOPMENT/APPROVAL
-
\r\n\r\n\r\nACQUISITION/PARTNERSHIP
-
\r\n\r\n\r\nLIST
-
Of figures
-
\r\n\r\n\r\nMARKET SYNOPSIS
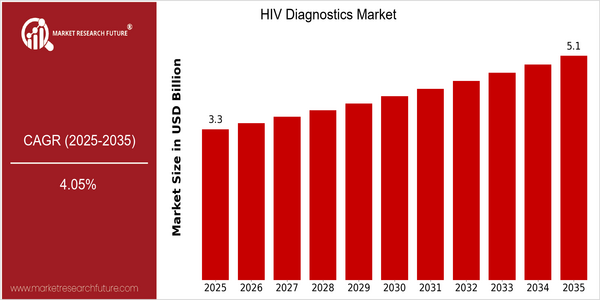
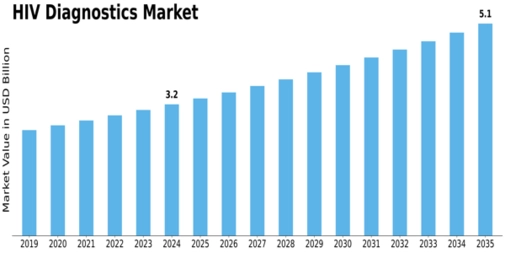
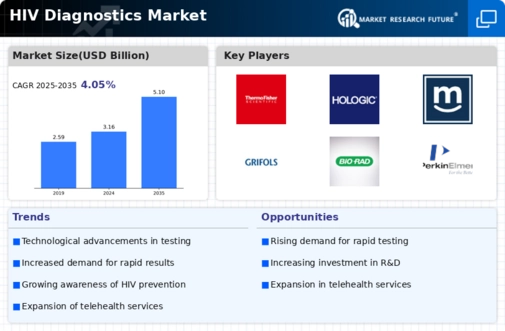
-
\r\n\r\n\r\nNORTH
-
AMERICA HIV DIAGNOSTICS MARKET ANALYSIS
-
\r\n\r\n\r\nUS HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nUS HIV DIAGNOSTICS MARKET
-
ANALYSIS BY END USER
-
\r\n\r\n\r\nUS HIV DIAGNOSTICS MARKET ANALYSIS
-
BY PRODUCT TYPE
-
\r\n\r\n\r\nUS HIV DIAGNOSTICS MARKET ANALYSIS BY
-
TECHNOLOGY
-
\r\n\r\n\r\nUS HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nCANADA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nCANADA HIV
-
DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nCANADA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nCANADA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nCANADA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nEUROPE HIV DIAGNOSTICS MARKET
-
ANALYSIS
-
\r\n\r\n\r\nGERMANY HIV DIAGNOSTICS MARKET ANALYSIS BY TEST
-
TYPE
-
\r\n\r\n\r\nGERMANY HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nGERMANY
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nGERMANY
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nGERMANY
-
HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nUK HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nUK HIV DIAGNOSTICS MARKET
-
ANALYSIS BY END USER
-
\r\n\r\n\r\nUK HIV DIAGNOSTICS MARKET ANALYSIS
-
BY PRODUCT TYPE
-
\r\n\r\n\r\nUK HIV DIAGNOSTICS MARKET ANALYSIS BY
-
TECHNOLOGY
-
\r\n\r\n\r\nUK HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nFRANCE
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nFRANCE HIV
-
DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nFRANCE HIV DIAGNOSTICS
-
MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nFRANCE HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nFRANCE HIV DIAGNOSTICS
-
MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nRUSSIA HIV DIAGNOSTICS MARKET
-
ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nRUSSIA HIV DIAGNOSTICS MARKET ANALYSIS
-
BY END USER
-
\r\n\r\n\r\nRUSSIA HIV DIAGNOSTICS MARKET ANALYSIS BY
-
PRODUCT TYPE
-
\r\n\r\n\r\nRUSSIA HIV DIAGNOSTICS MARKET ANALYSIS BY
-
TECHNOLOGY
-
\r\n\r\n\r\nRUSSIA HIV DIAGNOSTICS MARKET ANALYSIS BY
-
REGIONAL
-
\r\n\r\n\r\nITALY HIV DIAGNOSTICS MARKET ANALYSIS BY TEST
-
TYPE
-
\r\n\r\n\r\nITALY HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nITALY
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nITALY
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nITALY HIV
-
DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSPAIN HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nSPAIN HIV DIAGNOSTICS MARKET
-
ANALYSIS BY END USER
-
\r\n\r\n\r\nSPAIN HIV DIAGNOSTICS MARKET ANALYSIS
-
BY PRODUCT TYPE
-
\r\n\r\n\r\nSPAIN HIV DIAGNOSTICS MARKET ANALYSIS
-
BY TECHNOLOGY
-
\r\n\r\n\r\nSPAIN HIV DIAGNOSTICS MARKET ANALYSIS BY
-
REGIONAL
-
\r\n\r\n\r\nREST OF EUROPE HIV DIAGNOSTICS MARKET ANALYSIS
-
BY TEST TYPE
-
\r\n\r\n\r\nREST OF EUROPE HIV DIAGNOSTICS MARKET ANALYSIS
-
BY END USER
-
\r\n\r\n\r\nREST OF EUROPE HIV DIAGNOSTICS MARKET ANALYSIS
-
BY PRODUCT TYPE
-
\r\n\r\n\r\nREST OF EUROPE HIV DIAGNOSTICS MARKET
-
ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nREST OF EUROPE HIV DIAGNOSTICS
-
MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nAPAC HIV DIAGNOSTICS MARKET
-
ANALYSIS
-
\r\n\r\n\r\nCHINA HIV DIAGNOSTICS MARKET ANALYSIS BY TEST
-
TYPE
-
\r\n\r\n\r\nCHINA HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nCHINA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nCHINA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nCHINA HIV
-
DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nINDIA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nINDIA HIV DIAGNOSTICS MARKET
-
ANALYSIS BY END USER
-
\r\n\r\n\r\nINDIA HIV DIAGNOSTICS MARKET ANALYSIS
-
BY PRODUCT TYPE
-
\r\n\r\n\r\nINDIA HIV DIAGNOSTICS MARKET ANALYSIS
-
BY TECHNOLOGY
-
\r\n\r\n\r\nINDIA HIV DIAGNOSTICS MARKET ANALYSIS BY
-
REGIONAL
-
\r\n\r\n\r\nJAPAN HIV DIAGNOSTICS MARKET ANALYSIS BY TEST
-
TYPE
-
\r\n\r\n\r\nJAPAN HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nJAPAN
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nJAPAN
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nJAPAN HIV
-
DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSOUTH KOREA HIV
-
DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nSOUTH KOREA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nSOUTH KOREA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nSOUTH
-
KOREA HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nSOUTH
-
KOREA HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nMALAYSIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nMALAYSIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nMALAYSIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nMALAYSIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nMALAYSIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nTHAILAND
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nTHAILAND
-
HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nTHAILAND
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nTHAILAND
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nTHAILAND
-
HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nINDONESIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nINDONESIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nINDONESIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nINDONESIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nINDONESIA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nREST OF APAC
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nREST OF
-
APAC HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nREST
-
OF APAC HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nREST
-
OF APAC HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nREST
-
OF APAC HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSOUTH
-
AMERICA HIV DIAGNOSTICS MARKET ANALYSIS
-
\r\n\r\n\r\nBRAZIL HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nBRAZIL HIV DIAGNOSTICS MARKET
-
ANALYSIS BY END USER
-
\r\n\r\n\r\nBRAZIL HIV DIAGNOSTICS MARKET ANALYSIS
-
BY PRODUCT TYPE
-
\r\n\r\n\r\nBRAZIL HIV DIAGNOSTICS MARKET ANALYSIS
-
BY TECHNOLOGY
-
\r\n\r\n\r\nBRAZIL HIV DIAGNOSTICS MARKET ANALYSIS
-
BY REGIONAL
-
\r\n\r\n\r\nMEXICO HIV DIAGNOSTICS MARKET ANALYSIS BY
-
TEST TYPE
-
\r\n\r\n\r\nMEXICO HIV DIAGNOSTICS MARKET ANALYSIS BY END
-
USER
-
\r\n\r\n\r\nMEXICO HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT
-
TYPE
-
\r\n\r\n\r\nMEXICO HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nMEXICO
-
HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nARGENTINA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nARGENTINA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nARGENTINA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nARGENTINA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nARGENTINA
-
HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nREST OF SOUTH
-
AMERICA HIV DIAGNOSTICS MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA HIV DIAGNOSTICS MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA HIV DIAGNOSTICS MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA HIV DIAGNOSTICS MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nREST
-
OF SOUTH AMERICA HIV DIAGNOSTICS MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nMEA
-
HIV DIAGNOSTICS MARKET ANALYSIS
-
\r\n\r\n\r\nGCC COUNTRIES HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nGCC COUNTRIES HIV DIAGNOSTICS
-
MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nGCC COUNTRIES HIV DIAGNOSTICS
-
MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nGCC COUNTRIES HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nGCC COUNTRIES HIV DIAGNOSTICS
-
MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nSOUTH AFRICA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nSOUTH AFRICA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nSOUTH AFRICA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nSOUTH AFRICA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nSOUTH AFRICA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nREST OF MEA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TEST TYPE
-
\r\n\r\n\r\nREST OF MEA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY END USER
-
\r\n\r\n\r\nREST OF MEA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY PRODUCT TYPE
-
\r\n\r\n\r\nREST OF MEA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY TECHNOLOGY
-
\r\n\r\n\r\nREST OF MEA HIV DIAGNOSTICS
-
MARKET ANALYSIS BY REGIONAL
-
\r\n\r\n\r\nKEY BUYING CRITERIA OF HIV
-
DIAGNOSTICS MARKET
-
\r\n\r\n\r\nRESEARCH PROCESS OF MRFR
-
\r\n\r\n\r\nDRO
-
ANALYSIS OF HIV DIAGNOSTICS MARKET
-
\r\n\r\n\r\nDRIVERS IMPACT ANALYSIS:
-
HIV DIAGNOSTICS MARKET
-
\r\n\r\n\r\nRESTRAINTS IMPACT ANALYSIS: HIV
-
DIAGNOSTICS MARKET
-
\r\n\r\n\r\nSUPPLY / VALUE CHAIN: HIV DIAGNOSTICS
-
MARKET
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET, BY TEST TYPE, 2025 (%
-
SHARE)
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET, BY TEST TYPE, 2019 TO
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET, BY END USER,
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET, BY END USER, 2019
-
TO 2035 (USD Billions)
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET, BY PRODUCT
-
TYPE, 2025 (% SHARE)
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET, BY PRODUCT
-
TYPE, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET,
-
BY TECHNOLOGY, 2025 (% SHARE)
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET,
-
BY TECHNOLOGY, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nHIV DIAGNOSTICS
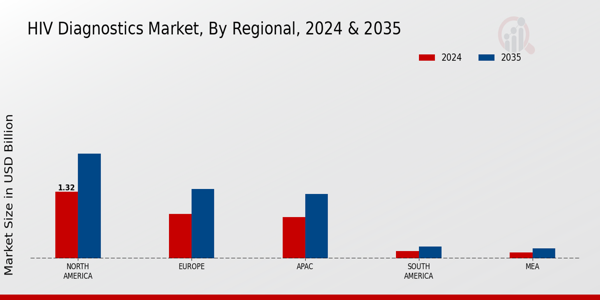
-
MARKET, BY REGIONAL, 2025 (% SHARE)
-
\r\n\r\n\r\nHIV DIAGNOSTICS MARKET,
-
BY REGIONAL, 2019 TO 2035 (USD Billions)
-
\r\n\r\n\r\nBENCHMARKING
-
OF MAJOR COMPETITORS
-
\r\n\r\n\r\n










Leave a Comment