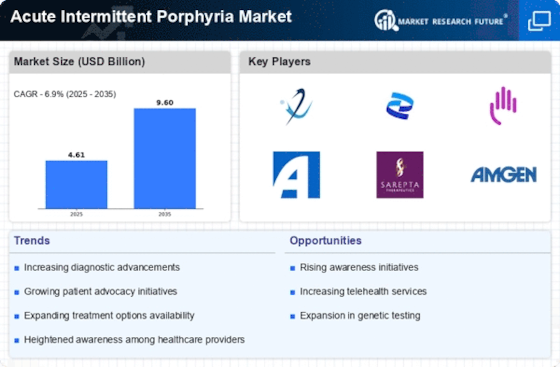
Top Industry Leaders in the Acute Intermittent Porphyria Market

Latest Acute Intermittent Porphyria Companies Updates:
Alnylam PharmaceuticalsPositive topline results from Phase 3 ENVISION study of givosiran, an investigational RNAi therapeutic for acute hepatic porphyria (including AIP).Givosiran granted Breakthrough Therapy Designation by the US FDA.Ongoing Phase 3 ENVISION2 study of givosiran for AIP prophylaxis.
Apellis Pharmaceuticals Development of APL-1202, a pegylated hemin product for the treatment of acute porphyria attacks.Phase 2b study of APL-1202 ongoing, with results expected in 2024.
Akcea TherapeuticsMarketing Lumitrio (givosiran) for the treatment of acute hepatic porphyria in the US and Europe.Expanding access to Lumitrio through patient assistance programs and collaborations with healthcare providers.
BioMarin PharmaceuticalMarketing Vyndaqel (tabeleumac) for the treatment of hereditary transthyretin-mediated amyloidosis (hATTR), which can have some overlapping symptoms with AIP.Continuing research and development efforts for other rare diseases, including porphyrias.
List of Acute Intermittent Porphyria Key companies in the market:
- ACON Laboratories, Inc. (US)
- Siemens AG (US)
- Dahaner (US)
- Bio-Rad Laboratories Inc. (US)
- Sysmex Corporation (Japan)
- Hoffmann-La Roche Ltd (Switzerland)
- ARKRAY Inc. (Japan)