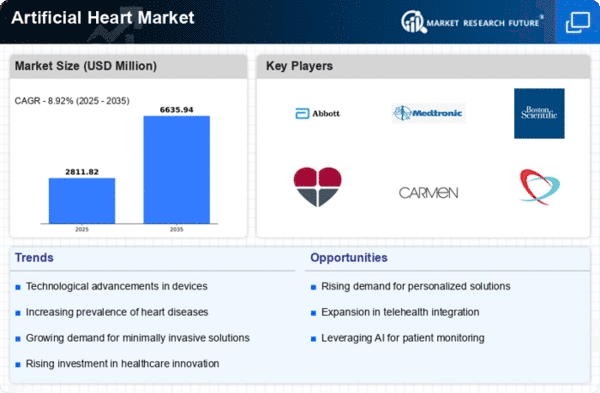
Top Industry Leaders in the Artificial Heart Market
 Latest Artificial heart Companies Update
Latest Artificial heart Companies Update
-
January 2023: Carmat, a French manufacturer of artificial hearts, anticipates reaching profitability by 2027, given the incremental resumption of implant operations and the robust demand it encounters in Europe prior to the product's commercial release in the United States. The manufacturer of Aeson implants, which aims to introduce the product in the United States in 2026, intends to increase production to 100 hearts in 2023, 500 hearts in 2024, and 1,000 hearts in 2027. Carmat disclosed for the first time its financial projections, informing investors and reporters that it anticipated the addressable market to surpass $40 billion by 2030, with more than 200,000 prospective patients annually in the United States and Europe. In excess of $1 billion in annual revenue could be generated for the business over the next decade.
- Eko Health announced the release of the CORE 500, a digital stethoscope of the next iteration, in July 2023. Engineered by Eko of California, the system was designed to improve diagnostic precision and early disease detection. It features a three-lead electrocardiogram (ECG), artificial intelligence (AI) software, high-fidelity audio, and a full-color display. According to Eko, the system provides a critical patient assessment instrument that is indispensable for healthcare practitioners. FDA approval was granted to CORE 500 last month. It provides compatibility with Eko's Sensora platform, which detects cardiac disease using artificial intelligence (AI). When combined with their robust AI algorithms, it has the capability to identify leading indicators of heart disease within seconds, providing significant support to healthcare practitioners in their endeavors to diagnose diverse cardiac conditions with heightened precision and assurance.
List ofArtificial Heart Key companies in the market
- SynCardia Systems, LLC
- BiVACOR Inc.
- CARMAT, Cleveland Heart, Inc.
- ABIOMED, CryoLife, Inc.
- Abbott
- Thoratec Corporation