Aging Population
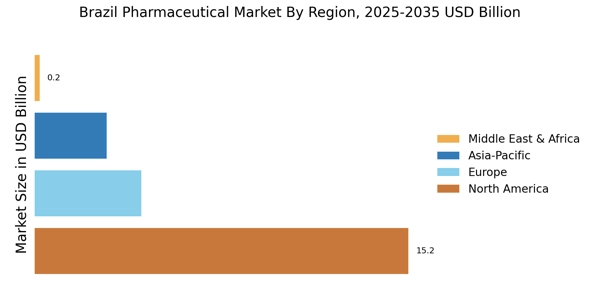
Brazil's demographic shift towards an aging population is significantly influencing the Brazil Pharmaceutical Industry Market. By 2025, it is estimated that over 30% of the Brazilian population will be aged 60 and above. This demographic change is associated with a higher prevalence of chronic diseases, such as diabetes and cardiovascular conditions, which require ongoing medication and treatment. Pharmaceutical companies are likely to focus on developing products tailored to the needs of older adults, thereby expanding their market presence. The increasing demand for age-related healthcare solutions is expected to drive innovation and growth within the Brazil Pharmaceutical Industry Market.
Expansion of Generic Drugs
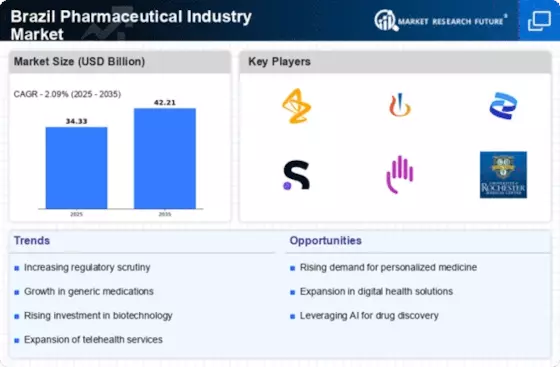
The Brazil Pharmaceutical Industry Market is witnessing a robust expansion of generic drugs, which are becoming increasingly popular due to their affordability and accessibility. The Brazilian government has implemented policies to promote the use of generics, resulting in a significant increase in their market share. As of 2025, generic drugs account for approximately 40% of the total pharmaceutical market in Brazil. This trend is likely to continue, as patients and healthcare providers increasingly favor cost-effective alternatives to branded medications. The growth of generics not only enhances competition but also contributes to the overall sustainability of the Brazil Pharmaceutical Industry Market.
Increasing Healthcare Expenditure
The Brazil Pharmaceutical Industry Market is experiencing a notable increase in healthcare expenditure, driven by both public and private sectors. In recent years, the Brazilian government has allocated a larger portion of its budget to healthcare, which has resulted in improved access to medications and treatments. As of 2025, healthcare spending in Brazil reached approximately 9% of GDP, reflecting a growing commitment to health services. This trend is likely to continue, as the government aims to enhance the quality of healthcare and expand coverage. Consequently, the increased funding is expected to stimulate demand for pharmaceutical products, thereby propelling growth within the Brazil Pharmaceutical Industry Market.
Government Initiatives for Local Production
The Brazilian government is actively promoting local production of pharmaceuticals to reduce dependency on imports and enhance self-sufficiency. Initiatives such as tax incentives and funding for local manufacturers are being implemented to stimulate domestic production. As of 2025, local production accounts for approximately 60% of the pharmaceutical market in Brazil, reflecting a significant shift towards homegrown solutions. This strategy not only supports the economy but also ensures a more stable supply of essential medications. The emphasis on local production is expected to bolster the Brazil Pharmaceutical Industry Market, fostering innovation and competitiveness.
Technological Advancements in Drug Development
Technological advancements are playing a crucial role in shaping the Brazil Pharmaceutical Industry Market. Innovations in biotechnology, artificial intelligence, and data analytics are streamlining drug development processes, reducing time-to-market for new therapies. Brazilian pharmaceutical companies are increasingly adopting these technologies to enhance research and development capabilities. As of 2025, it is projected that investments in technology-driven drug development will increase by 15% annually. This trend suggests that the Brazil Pharmaceutical Industry Market is likely to witness a surge in novel therapies and improved treatment options, ultimately benefiting patients and healthcare providers alike.