Growing Awareness and Advocacy
The growing awareness and advocacy surrounding Bulbospinal Muscular Atrophy are driving forces in the Bulbospinal Muscular Atrophy Drug Market. Increased efforts by patient advocacy groups and healthcare organizations are raising public consciousness about BMA, its symptoms, and the need for effective treatments. This heightened awareness is likely to lead to earlier diagnoses and increased demand for therapeutic options. Furthermore, advocacy initiatives are fostering collaboration between stakeholders, including researchers, healthcare providers, and pharmaceutical companies, to address the unmet needs of patients. As awareness continues to grow, it is anticipated that the Bulbospinal Muscular Atrophy Drug Market will witness a surge in interest and investment, ultimately benefiting patients and their families.
Advancements in Genetic Research
Recent advancements in genetic research are transforming the landscape of the Bulbospinal Muscular Atrophy Drug Market. The identification of specific genetic mutations associated with BMA has opened new avenues for targeted therapies. For instance, the discovery of the SMN1 gene's role in the disease has led to the development of gene therapies that aim to address the underlying genetic causes. This shift towards precision medicine is likely to enhance treatment efficacy and patient outcomes. Furthermore, the integration of genetic testing into clinical practice is expected to facilitate early diagnosis and personalized treatment plans, thereby driving market growth. As research continues to evolve, the potential for innovative therapies based on genetic insights will likely reshape the Bulbospinal Muscular Atrophy Drug Market.
Increased Funding for Rare Disease Research
The surge in funding for rare disease research is a significant catalyst for the Bulbospinal Muscular Atrophy Drug Market. Governments and private organizations are increasingly recognizing the need to support research initiatives aimed at developing treatments for rare conditions like BMA. In recent years, funding allocations have seen a marked increase, with billions of dollars directed towards research and development. This financial support is crucial for fostering innovation and accelerating the clinical development of new therapies. Additionally, collaborations between academic institutions and pharmaceutical companies are becoming more prevalent, further enhancing the research landscape. As funding continues to grow, it is anticipated that the Bulbospinal Muscular Atrophy Drug Market will experience substantial advancements in therapeutic options.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly providing support for the development of innovative therapies in the Bulbospinal Muscular Atrophy Drug Market. Initiatives such as accelerated approval pathways and orphan drug designations are designed to expedite the availability of new treatments for rare diseases. These regulatory incentives encourage pharmaceutical companies to invest in the development of novel therapies for BMA, which may otherwise be deemed unprofitable. The presence of a favorable regulatory environment is likely to enhance the attractiveness of the market for investors and developers alike. As more therapies receive regulatory approval, the market is expected to expand, offering patients access to a broader range of treatment options. This supportive regulatory framework is poised to play a crucial role in shaping the future of the Bulbospinal Muscular Atrophy Drug Market.
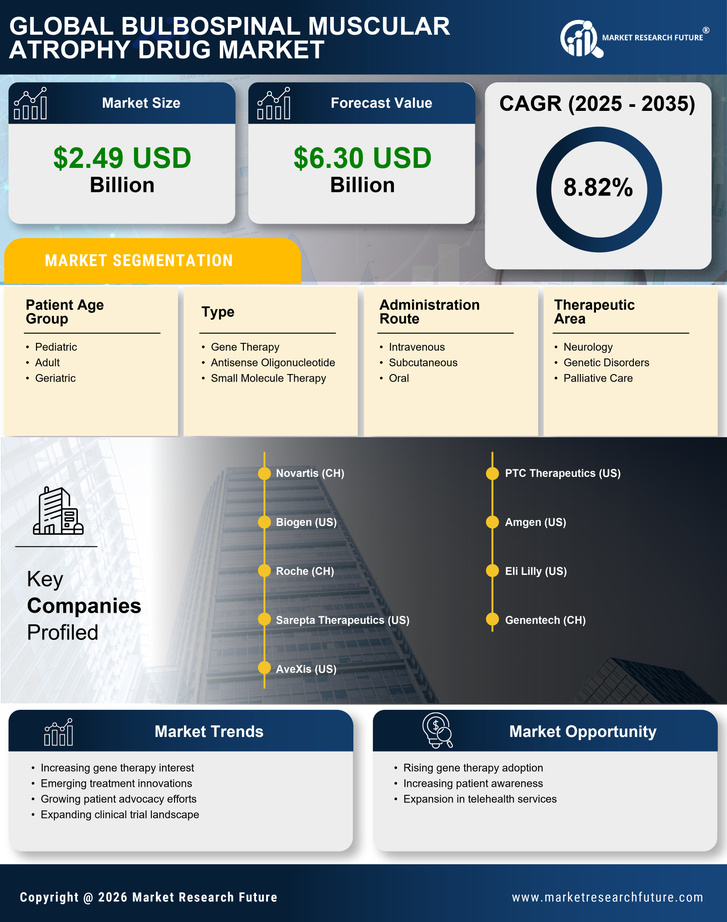
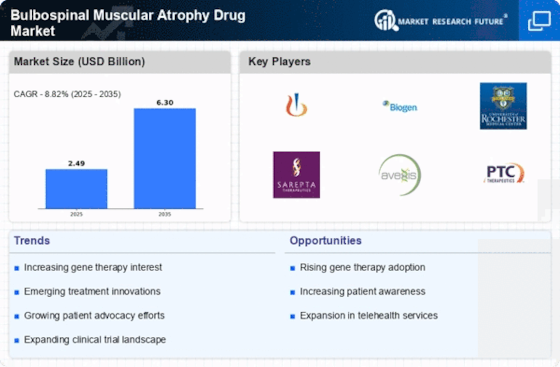
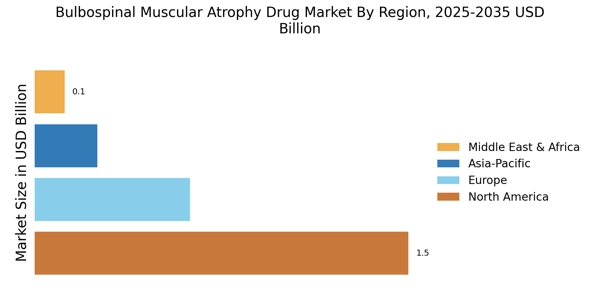
Rising Prevalence of Bulbospinal Muscular Atrophy
The increasing incidence of Bulbospinal Muscular Atrophy (BMA) is a pivotal driver for the Bulbospinal Muscular Atrophy Drug Market. Recent estimates suggest that the prevalence of BMA ranges from 1 in 10,000 to 1 in 25,000 individuals, depending on the population studied. This rising prevalence necessitates the development of effective therapeutic options, thereby stimulating market growth. As awareness of BMA expands, more individuals are being diagnosed, which further propels the demand for innovative treatments. The growing patient population is likely to attract pharmaceutical companies to invest in research and development, ultimately enhancing the therapeutic landscape for BMA. Consequently, the increasing prevalence of this condition is expected to significantly influence the dynamics of the Bulbospinal Muscular Atrophy Drug Market.