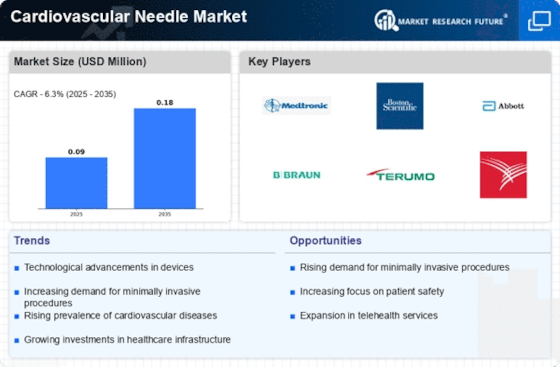
Top Industry Leaders in the Cardiovascular Needle Market
 Latest Cardiovascular Needle Companies Update
Latest Cardiovascular Needle Companies Update
-
June 2023: The FDA has granted 510(k) authorization for MR Conditional labeling of the Arrow EZ-IO Needle, the first and only intraosseous (IO) needle, according to a recent announcement by Teleflex Corporation TFX. An essential component of the Arrow EZ-IO Intraosseous Vascular Access System, the EZ-IO Needle's innovative diamond point is designed to facilitate rapid, accurate, and stable insertion. The recent advancement will strengthen the Vascular Access division of Teleflex. When intravenous access is difficult to obtain as a result of an emergency, emergent situation, or medical necessity, the EZ-IO System may be of assistance. With the new labeling, the practitioner can continue treating MRI-required patients without interfering with the operation of the vascular access site. Clinical and Medical Affairs is committed to advocating for the broader utilization of Teleflex medical devices in order to optimize patient care.
-
November 2023: BD announced the launch of a new needleless blood draw technology that has received FDA approval. The introduction of the technology facilitates the "One-Stick Hospital Stay" initiative of the organization. Over two years ago, BD unveiled this endeavor with the intention of revolutionizing the patient experience. With 510(k) approval from the FDA, BD is now able to market its new needleless blood collection device, PIVO Pro. It incorporates design enhancements that enable it to function initially and exclusively with lengthy peripheral IV catheters that are integrated. The Nexiva closed IV catheter system with NearPort IV access is included, which further enhances the existing compatibility features. Since 2017, PIVO has been accessible via conventional brief peripheral IV catheters. The new solution, according to BD, integrates systems with the demonstrated clinical benefits.
List of Cardiovascular Needle Key companies in the market
- Becton, Dickinson, and Company (US)
- B. Braun Melsungen AG (Germany)
- Sheffield Ltd. (UK)
- CP Medical, Inc. (the US)
- Ethicon Inc. (US)
- KLS Martin Group (US)
- Sklar Surgical Instruments (US)
- Rumex International Corporation Ltd. (US)
- Scanlan International Inc. (US)
- Teleflex Incorporated (US)
- Medline Industries, Inc. (US)
- Surgins surgical Ltd. (UK)
- Surtex Instruments Ltd. (UK)
- Cardivon Surgical Inc. (China)
- Symmetry Surgical Inc. (US)
- Delacroix-Chevalier (France)
- Wexler Surgical (US)
- Quality Needles Pvt. Ltd. (India)
- FSSB surgical needles GmbH (Germany)