Advancements in Targeted Therapies
Recent advancements in targeted therapies are significantly influencing the Castrate Resistant Prostate Cancer Therapeutic Market. The development of drugs that specifically target molecular pathways involved in prostate cancer progression has shown promise in improving patient outcomes. For instance, therapies such as androgen receptor inhibitors and PARP inhibitors have emerged as effective options for patients with castrate-resistant prostate cancer. The market for these targeted therapies is projected to grow, with estimates indicating a compound annual growth rate of over 10% in the coming years. This growth is driven by ongoing clinical trials and research efforts aimed at identifying new targets and combinations that enhance therapeutic efficacy. As more targeted therapies receive regulatory approval, the Castrate Resistant Prostate Cancer Therapeutic Market is likely to witness a shift towards personalized treatment approaches, further driving market expansion.
Rising Investment in Cancer Research
The increasing investment in cancer research is a crucial driver for the Castrate Resistant Prostate Cancer Therapeutic Market. Governments and private organizations are allocating substantial funds to research initiatives aimed at understanding the biology of prostate cancer and developing novel treatment strategies. In recent years, funding for prostate cancer research has seen a notable increase, with billions of dollars being invested annually. This influx of capital supports clinical trials, drug development, and innovative research projects that focus on castrate-resistant prostate cancer. As a result, the market is likely to benefit from the introduction of new therapies and treatment modalities that emerge from these research efforts. The commitment to advancing cancer research not only enhances the understanding of the disease but also fosters collaboration among academic institutions, pharmaceutical companies, and healthcare providers, thereby propelling the Castrate Resistant Prostate Cancer Therapeutic Market forward.
Increasing Incidence of Prostate Cancer
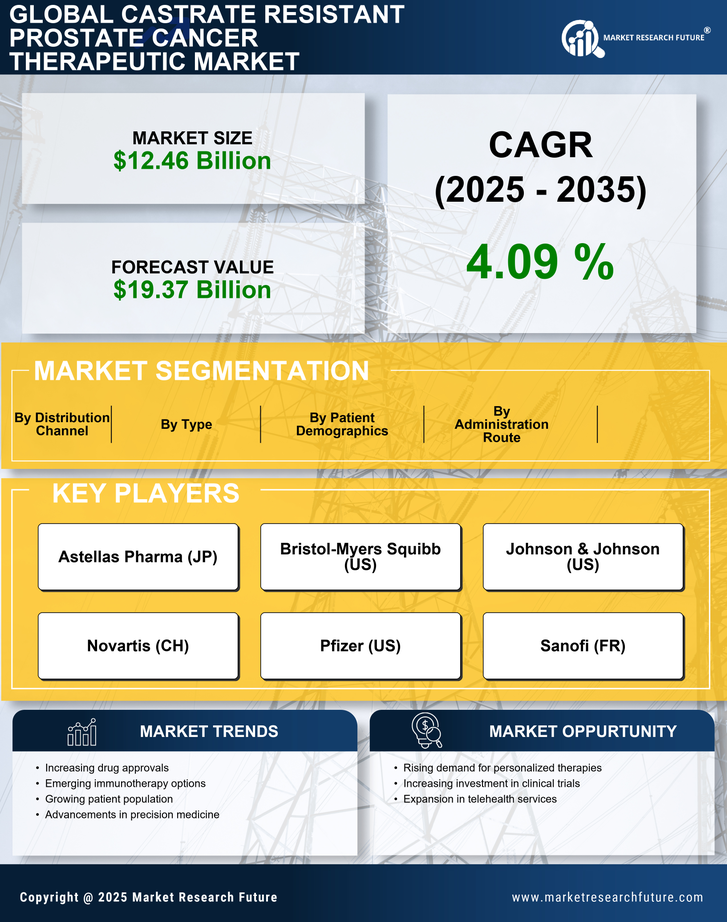
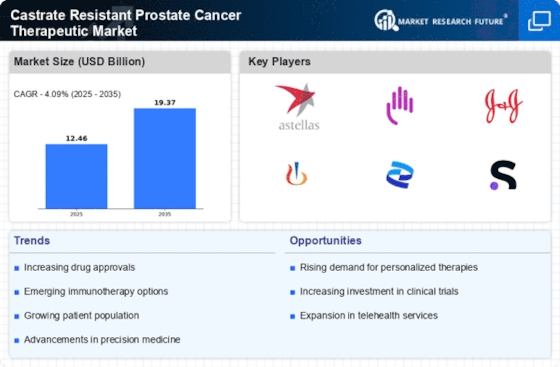
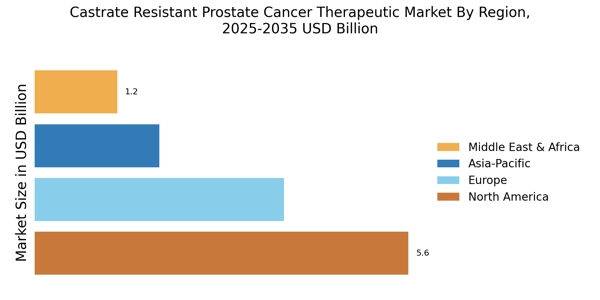
The rising incidence of prostate cancer is a pivotal driver for the Castrate Resistant Prostate Cancer Therapeutic Market. As the population ages, the prevalence of prostate cancer is expected to increase, with estimates suggesting that nearly 1 in 8 men will be diagnosed with the disease during their lifetime. This growing patient population necessitates the development of effective therapeutic options, particularly for castrate-resistant cases, which are more challenging to treat. The increasing number of diagnosed cases is likely to drive demand for innovative therapies, thereby expanding the market. Furthermore, advancements in diagnostic techniques are leading to earlier detection, which may contribute to a higher number of patients seeking treatment for advanced stages of the disease. Consequently, the Castrate Resistant Prostate Cancer Therapeutic Market is poised for growth as healthcare providers seek to address this escalating need.
Growing Awareness and Screening Programs
The growing awareness of prostate cancer and the implementation of screening programs are vital factors driving the Castrate Resistant Prostate Cancer Therapeutic Market. Increased public awareness campaigns have led to a greater understanding of the disease, encouraging men to seek early screening and diagnosis. As a result, more cases of prostate cancer are being identified at earlier stages, which can lead to a higher incidence of castrate-resistant cases as the disease progresses. Screening programs, such as PSA testing, have become more prevalent, contributing to the early detection of prostate cancer. This trend is likely to result in a larger patient population requiring advanced therapeutic options, thereby stimulating growth in the market. The emphasis on awareness and screening not only aids in early diagnosis but also highlights the need for effective treatments within the Castrate Resistant Prostate Cancer Therapeutic Market.
Regulatory Support for Innovative Therapies
Regulatory support for innovative therapies is an essential driver for the Castrate Resistant Prostate Cancer Therapeutic Market. Regulatory agencies are increasingly recognizing the need for expedited approval processes for breakthrough therapies that address unmet medical needs in prostate cancer treatment. Initiatives such as the FDA's Breakthrough Therapy Designation have facilitated faster access to promising new treatments for patients with castrate-resistant prostate cancer. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, leading to a more robust pipeline of innovative therapies. As new drugs receive swift approval, the market is likely to expand, providing patients with more options for managing their condition. The proactive stance of regulatory bodies in fostering innovation is expected to play a significant role in shaping the future of the Castrate Resistant Prostate Cancer Therapeutic Market.