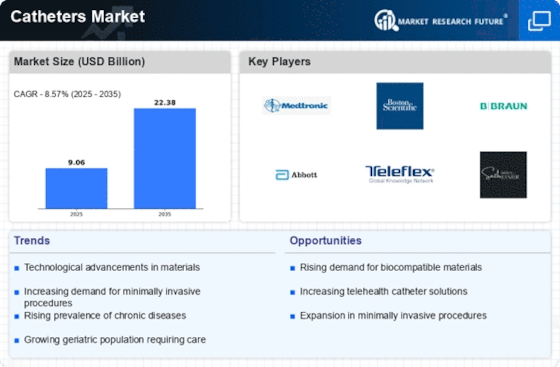
Top Industry Leaders in the Catheters Market
Catheters_Key_Companies.jpg" alt="Catheters Key Companies" width="600" height="300">Disclaimer: List of key companies in no particular orderLatest Catheters Companies Update
-
October 2023: According to Shockwave Medical, a novel coronary intravascular lithotripsy (IVL) catheter has been introduced in the United States. After receiving FDA approval, the Shockwave C2+ coronary IVL catheter is scheduled to be introduced. Compared to the Shockwave C2 catheter that the FDA authorized in June 2021, this catheter gives 50% more pulses per catheter. An further benefit of IVL for treating complicated calcium ideally will be the improvements of Shockwave C2+, which will help with nodular and thick, eccentric calcium as well as more diffuse illness. The upgraded catheter can increase workflow and procedural efficiency when more pulses are added to the intuitive catheter design and user-friendly system that are fundamental to Shockwave IVL's success.
-
October 2023: The new Introcan Safety® 2 IV Catheter with Multi-Access Blood Control was introduced today by B. Braun Medical Inc., a pioneer in smart infusion treatment. The most recent addition to B. Braun's extensive range of passive needlestick avoidance catheters is the Introcan Safety 2 IV Catheter. Every time the hub is accessed, a blood control function protects physicians from blood exposure in addition to automated needlestick protection, lowering the risk of exposure to bloodborne pathogens throughout the IV procedure. An essential component of infusion treatment is IV access, and needlestick injuries are still a major danger that doctors must deal with on a regular basis, putting them at risk for exposure to bloodborne infections. According to studies, fully automated passive safety devices reduce needlestick injuries three times better than manually sliding shields and twice as well as semi-automatic "push button" safety shields.
List of Catheters Key companies in the market
- Boston Scientific Corporation,
- Koninklijke Philips N.V,
- Abbott, Medtronic Plc,
- Braun Melsungen Ag,
- Becton, Dickinson, And Company,
- Terumo Corporation,
- Lumend Corporation
- Covidien Ag (Ireland),
- Acist Medical Systems
- Cook Medical Inc.