Market Share
Catheters Market Share Analysis
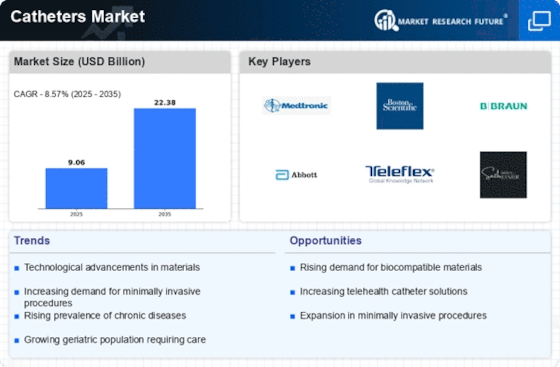
The catheters market is growing due to an aging population, rising cardiovascular disease rates, and medical technology. To develop a strong presence and obtain a competitive advantage in catheter solutions, catheter firms use several market share positioning tactics. Leading catheter manufacturers focus technology and product innovation. Advanced catheters with increased flexibility, materials, and designs are developed via research and development. Technology helps attract healthcare professionals and patients, increasing market share. Market share positioning requires partnerships with healthcare institutions, medical practitioners, and industry. These relationships promote clinical insights, information sharing, and research collaboration. Strong healthcare ecosystem partnerships boost a company's reputation and market share. Catheter firms strategically expand into new areas. This requires knowing local healthcare demands, meeting varied regulatory requirements, and creating effective distribution networks. Global presence helps organizations enter developing areas and broaden their client base, increasing market share. Education and training are crucial for catheter market share. Companies educate healthcare personnel about catheter usage and advantages. By educating practitioners, corporations increase catheter usage and position themselves as industry leaders. In price-sensitive markets, cost competitiveness affects market share. Catheter firms optimize manufacture, save costs, and provide competitive price. Affordable catheter solutions attract more customers, especially budget-conscious healthcare organizations, increasing market share. Medical device manufacturers must follow strict regulations and quality assurance criteria. Catheter manufacturers focus regulatory compliance to assure product safety and effectiveness. Quality increases healthcare professionals' and patients' trust, increasing market share. Patients have unique demands, hence catheter businesses provide customisation. Catheters tailored to medical conditions and patient needs improve consumer satisfaction. Patient-centric techniques boost market share via good experiences and recommendations. Continuous R&D investment is key to catheter market share positioning. Companies fund research into novel materials, technologies, and production methods. Innovative organizations may handle growing healthcare trends and gain market share by staying ahead. Reaching healthcare professionals and patients requires a strong digital presence. Catheter firms use internet advertising, social networking, and instructive material. Brand exposure, credibility, and market share rise with an efficient online presence. Customer satisfaction requires thorough post-insertion support and services. Companies provide healthcare professionals and patients with continual education, maintenance, and efficient customer support. Providing solid post-insertion service boosts customer loyalty and market share.

















Leave a Comment