Growing Awareness of Patient Safety
There is a growing emphasis on patient safety within healthcare systems, which serves as a significant driver for the Cerebral Oximetry Monitoring Market. Healthcare professionals are increasingly aware of the risks associated with inadequate monitoring of cerebral oxygen levels during surgical procedures and critical care. This awareness has led to the adoption of cerebral oximetry as a standard practice in many institutions. The technology allows for continuous monitoring, thereby reducing the likelihood of adverse events related to cerebral hypoxia. As hospitals and surgical centers strive to enhance patient safety protocols, the demand for cerebral oximetry solutions is expected to rise. This trend is further supported by regulatory bodies advocating for improved monitoring practices, which could potentially lead to increased market penetration of cerebral oximetry devices.
Rising Demand in Surgical Procedures
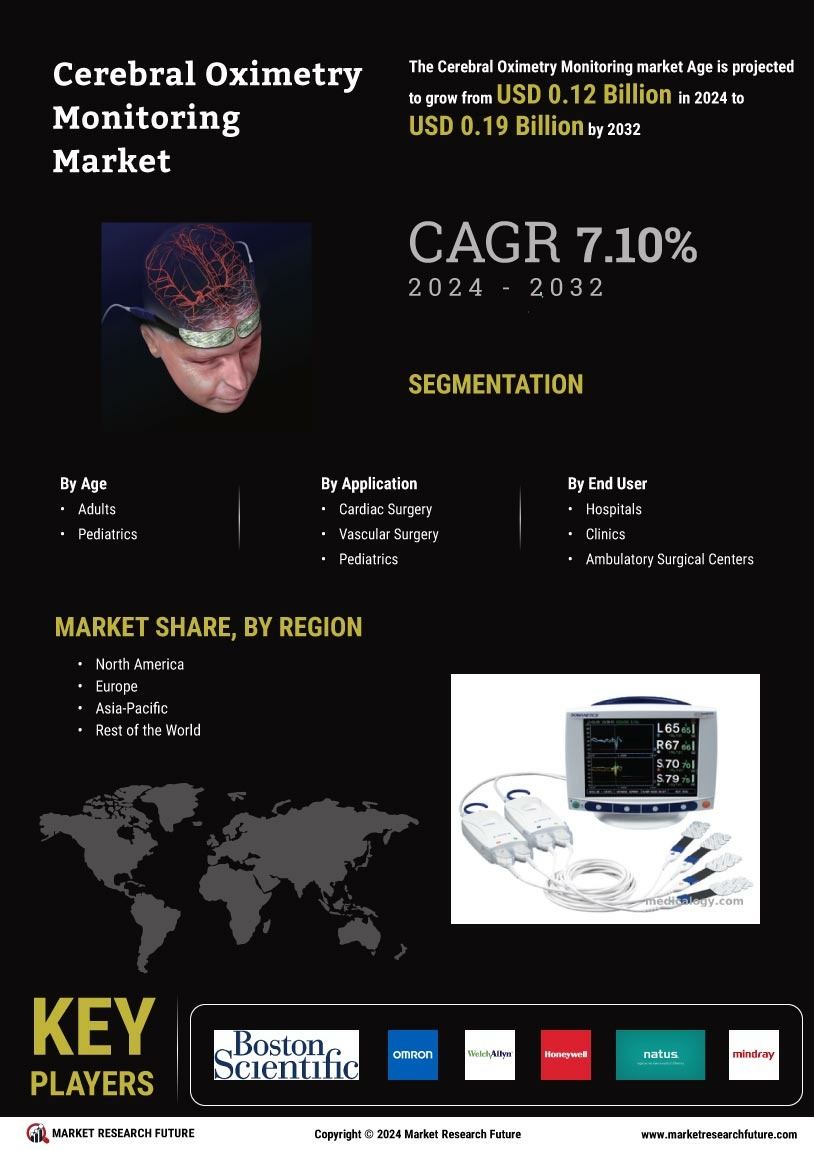
The increasing number of surgical procedures worldwide is a notable driver for the Cerebral Oximetry Monitoring Market. As surgical techniques become more complex, the need for precise monitoring of cerebral oxygenation during operations is paramount. Cerebral oximetry provides critical insights that can influence surgical outcomes, particularly in high-risk patients. Market analysis reveals that the surgical segment is one of the fastest-growing areas for cerebral oximetry applications, with a notable increase in its adoption in neurosurgery and cardiac surgery. This trend is likely to continue as healthcare providers seek to enhance patient safety and optimize surgical results. The integration of cerebral oximetry into standard surgical protocols may become more prevalent, further solidifying its role in modern surgical practice.
Expansion of Healthcare Infrastructure
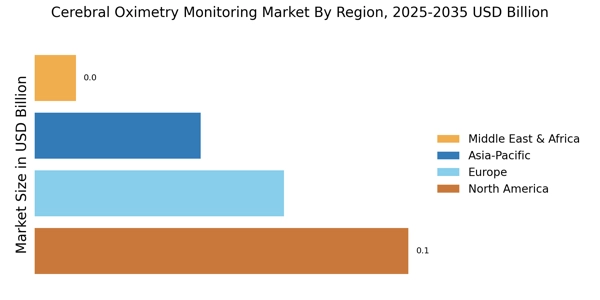
The expansion of healthcare infrastructure in emerging markets is a significant driver for the Cerebral Oximetry Monitoring Market. As countries invest in healthcare facilities and technologies, the demand for advanced monitoring solutions, including cerebral oximetry, is expected to rise. This expansion is particularly evident in regions where access to quality healthcare is improving, leading to increased surgical volumes and critical care needs. Market data suggests that investments in healthcare infrastructure are correlated with the adoption of innovative monitoring technologies. As hospitals and clinics upgrade their capabilities, the integration of cerebral oximetry into their monitoring systems becomes more likely. This trend indicates a promising future for the market, as enhanced healthcare infrastructure supports the growth of cerebral oximetry solutions.
Increasing Prevalence of Neurological Disorders
The rising incidence of neurological disorders, such as stroke and traumatic brain injury, is a key driver for the Cerebral Oximetry Monitoring Market. As these conditions become more prevalent, the demand for effective monitoring solutions intensifies. Cerebral oximetry provides real-time data on cerebral oxygenation, which is crucial for timely interventions. According to recent estimates, the number of stroke cases is projected to increase significantly, leading to a heightened need for advanced monitoring technologies. This trend suggests that healthcare providers are increasingly recognizing the importance of cerebral oximetry in improving patient outcomes, thereby propelling market growth. Furthermore, the integration of cerebral oximetry with other monitoring systems enhances its utility, making it an essential tool in critical care settings.
Technological Innovations in Monitoring Devices
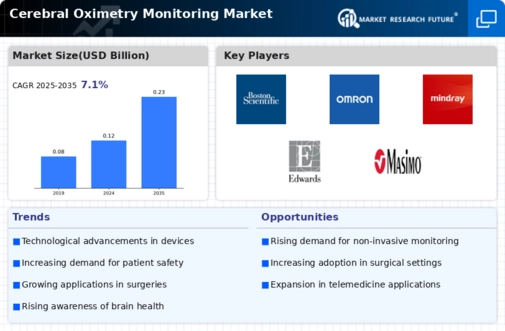
Technological advancements in cerebral oximetry devices are driving the growth of the Cerebral Oximetry Monitoring Market. Innovations such as wireless connectivity, improved sensor accuracy, and user-friendly interfaces are enhancing the functionality and appeal of these devices. The introduction of portable and non-invasive monitoring solutions is particularly noteworthy, as they allow for greater flexibility in patient management. Market data indicates that the segment of portable cerebral oximeters is experiencing rapid growth, reflecting a shift towards more accessible monitoring options. These innovations not only improve the accuracy of cerebral oxygenation readings but also facilitate better integration with electronic health records, thereby streamlining clinical workflows. As technology continues to evolve, the market for cerebral oximetry is likely to expand, driven by the demand for more sophisticated monitoring solutions.