Rising Incidence of Cancer
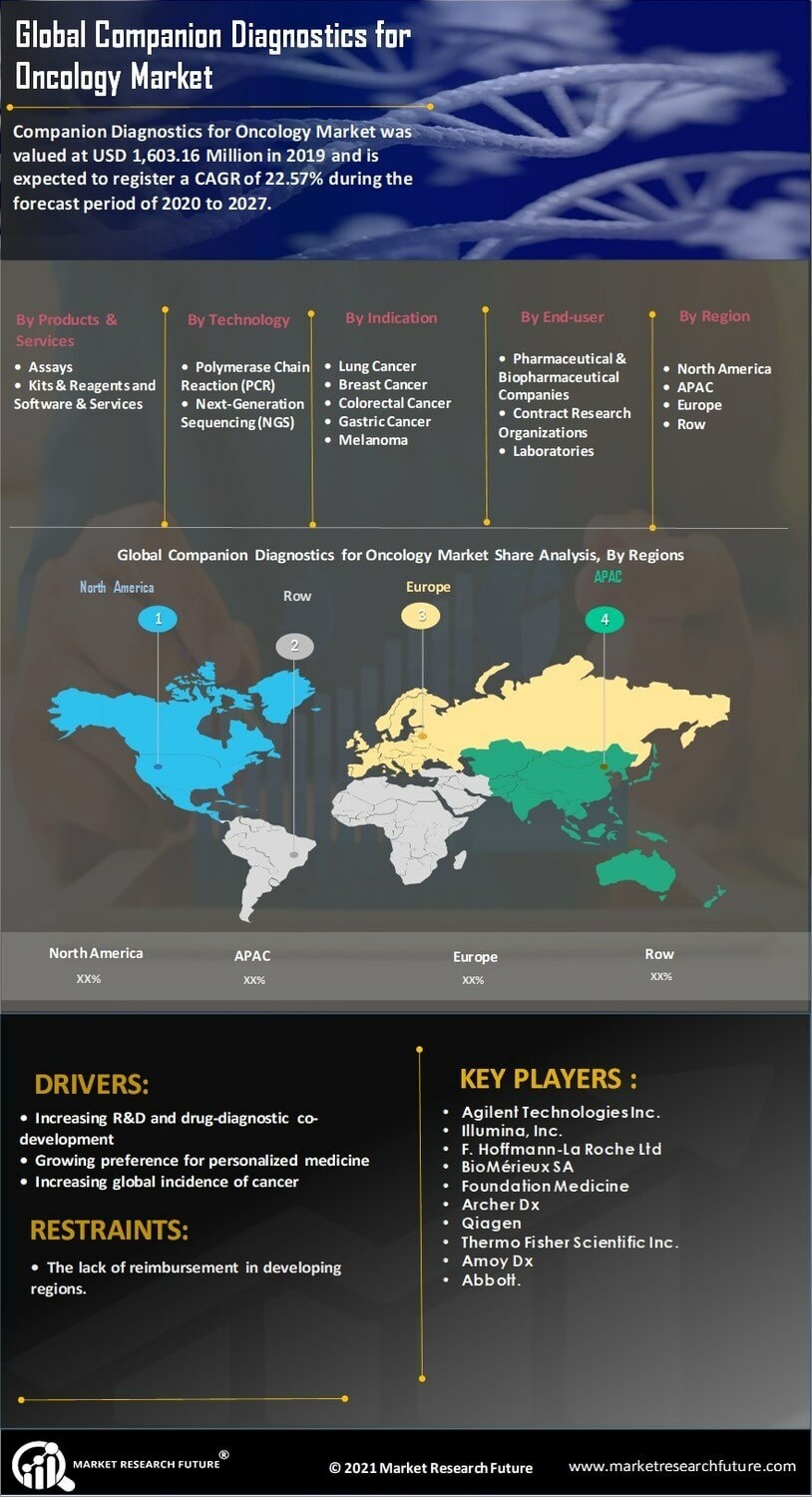
The increasing prevalence of cancer worldwide is a primary driver for the Companion Diagnostics for Oncology Market. As cancer cases rise, the demand for personalized treatment options intensifies. According to recent statistics, cancer is projected to affect millions annually, necessitating advanced diagnostic tools to tailor therapies effectively. Companion diagnostics play a crucial role in identifying suitable patients for targeted therapies, thereby enhancing treatment outcomes. This trend is likely to continue, as healthcare systems increasingly prioritize precision medicine. The Companion Diagnostics for Oncology Market is expected to expand significantly, driven by the urgent need for effective cancer management solutions.
Advancements in Genomic Technologies
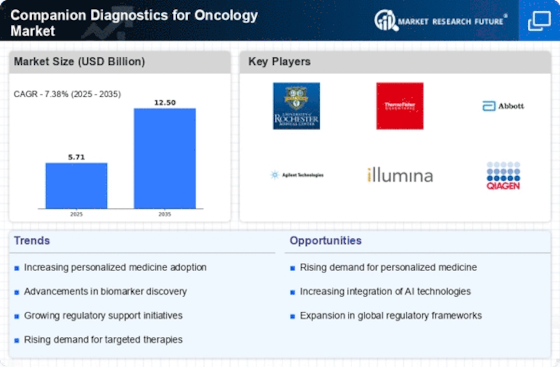
Technological innovations in genomics are propelling the Companion Diagnostics for Oncology Market forward. The advent of next-generation sequencing (NGS) and other genomic technologies has revolutionized cancer diagnostics, enabling the identification of specific biomarkers associated with various tumors. These advancements facilitate the development of companion diagnostics that can predict patient responses to targeted therapies. As a result, the market is witnessing a surge in the adoption of these technologies, with estimates suggesting a substantial increase in market value over the next few years. The integration of genomic data into clinical practice is likely to enhance the precision of cancer treatments, further driving the Companion Diagnostics for Oncology Market.
Increased Investment in Cancer Research
Investment in cancer research is a critical driver for the Companion Diagnostics for Oncology Market. Governments, private organizations, and pharmaceutical companies are allocating substantial resources to oncology research, aiming to discover new therapies and improve existing ones. This influx of funding supports the development of innovative companion diagnostics that can enhance treatment efficacy and patient selection. As research initiatives expand, the Companion Diagnostics for Oncology Market is likely to benefit from the resulting advancements in diagnostic technologies and therapeutic strategies. The ongoing commitment to cancer research suggests a promising future for this market.
Growing Demand for Personalized Medicine
The shift towards personalized medicine is a significant factor influencing the Companion Diagnostics for Oncology Market. Patients and healthcare providers increasingly seek tailored treatment options that consider individual genetic profiles. This trend is supported by a growing body of evidence indicating that personalized therapies can lead to improved patient outcomes. The Companion Diagnostics for Oncology Market is responding to this demand by developing tests that match patients with the most effective therapies based on their unique genetic makeup. As the healthcare landscape evolves, the emphasis on personalized medicine is expected to drive further growth in this market segment.
Regulatory Support for Companion Diagnostics
Regulatory bodies are increasingly recognizing the importance of companion diagnostics in oncology, providing a supportive framework for their development and approval. Streamlined regulatory processes and guidelines are facilitating the introduction of new diagnostic tests into the market. This regulatory support is crucial for the Companion Diagnostics for Oncology Market, as it encourages innovation and ensures that effective diagnostics reach patients in a timely manner. As regulatory environments continue to evolve, the market is expected to experience growth driven by the introduction of novel companion diagnostics that align with regulatory standards.