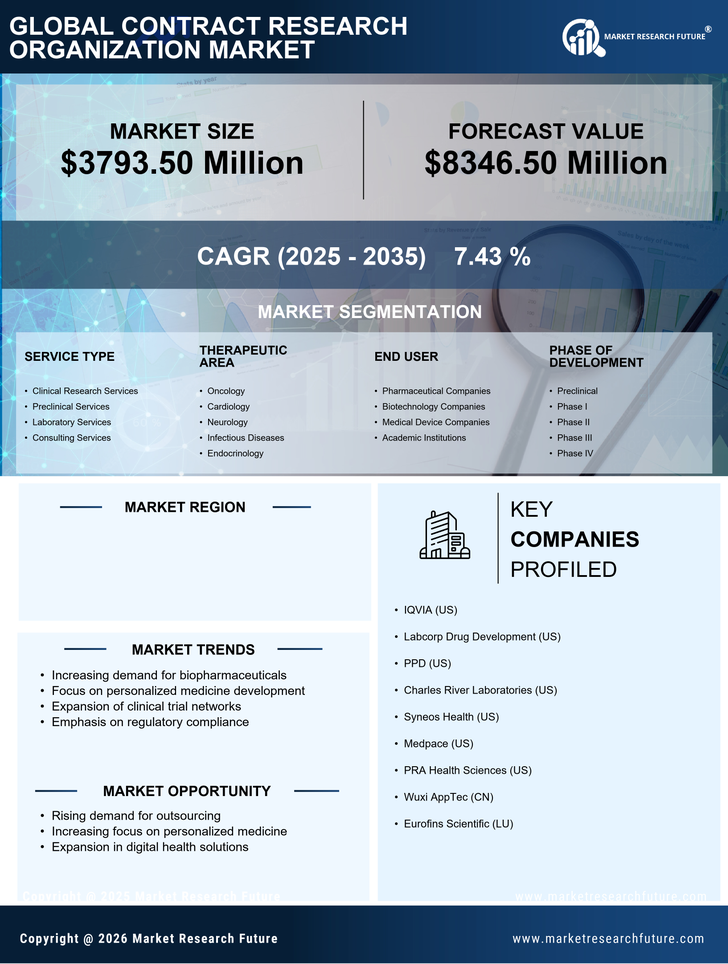
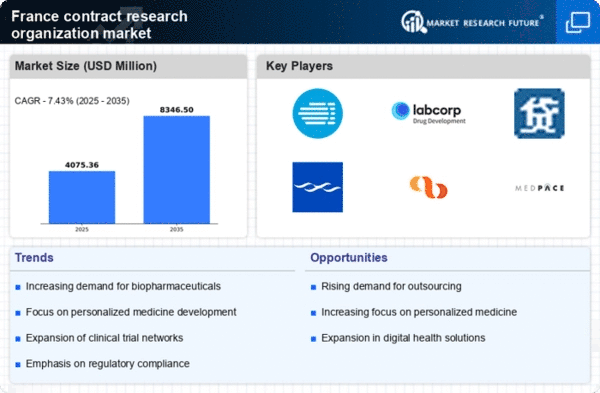
Increasing Investment in R&D
The contract research-organization market in France is experiencing a notable increase in investment in research and development (R&D). This trend is driven by both public and private sectors aiming to enhance innovation and competitiveness. In 2025, R&D expenditure in France is projected to reach approximately €70 billion, reflecting a growth of around 5% from the previous year. This influx of funding is likely to bolster the capabilities of contract research organizations, enabling them to offer more comprehensive services. As pharmaceutical and biotechnology companies seek to streamline their development processes, the demand for specialized services provided by contract research organizations is expected to rise, thereby propelling the market forward.
Focus on Personalized Medicine
The shift towards personalized medicine is significantly influencing the contract research-organization market in France. As healthcare becomes increasingly tailored to individual patient needs, the demand for clinical trials that support personalized therapies is growing. In 2025, it is estimated that around 30% of clinical trials in France will focus on personalized medicine, necessitating the expertise of contract research organizations. These organizations are well-positioned to assist in the design and execution of complex trials that require advanced methodologies and data analytics. This trend not only enhances patient outcomes but also drives the need for innovative solutions within the contract research-organization market.
Growing Demand for Data Analytics
The increasing importance of data analytics in clinical research is shaping the contract research-organization market in France. As the volume of data generated from clinical trials continues to rise, organizations are seeking advanced analytical solutions to derive meaningful insights. In 2025, it is projected that the demand for data analytics services within the contract research-organization market will increase by approximately 15%. This trend indicates a shift towards data-driven decision-making, where contract research organizations are expected to leverage sophisticated analytics tools to enhance trial efficiency and outcomes. Consequently, this growing demand for data analytics is likely to drive innovation and competitiveness within the market.
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector in France is expanding rapidly, which is positively impacting the contract research-organization market. With an increasing number of biopharmaceutical companies emerging, the demand for specialized research services is on the rise. In 2025, the biopharmaceutical market in France is projected to grow by approximately 8%, leading to a heightened need for contract research organizations to support drug development processes. This growth is likely to create opportunities for collaboration between biopharmaceutical firms and contract research organizations, facilitating the development of new therapies and enhancing the overall market landscape.
Regulatory Compliance and Quality Assurance
Regulatory compliance remains a critical driver for the contract research-organization market in France. As the regulatory landscape evolves, organizations must ensure adherence to stringent guidelines to maintain quality and safety in clinical trials. In 2025, it is anticipated that regulatory bodies will implement new standards that could affect up to 40% of ongoing trials. This necessitates the expertise of contract research organizations, which are equipped to navigate complex regulatory requirements. By providing quality assurance and compliance services, these organizations play a vital role in ensuring that clinical trials meet the necessary standards, thereby fostering trust and reliability in the market.