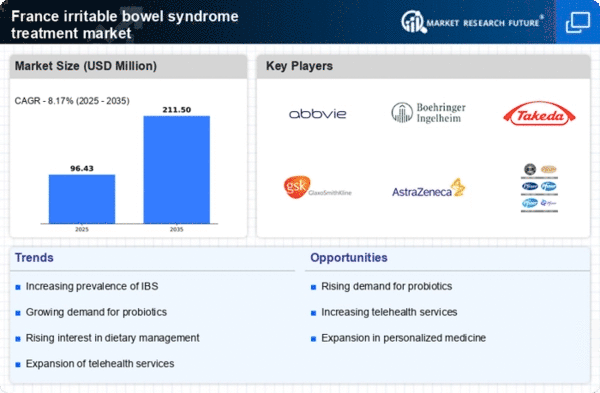
Rising Prevalence of IBS
The increasing prevalence of irritable bowel syndrome (IBS) in France is a significant driver for the irritable bowel-syndrome-treatment market. Recent studies indicate that approximately 10-15% of the French population experiences IBS symptoms, leading to a growing demand for effective treatment options. This rise in cases is attributed to various factors, including dietary changes and heightened stress levels. As more individuals seek medical attention for their symptoms, healthcare providers are compelled to offer a wider range of treatment modalities. Consequently, pharmaceutical companies are investing in research and development to create innovative therapies tailored to the unique needs of IBS patients. This trend not only enhances patient outcomes but also stimulates market growth, as the demand for specialized treatments continues to escalate.
Increased Focus on Patient Education
The irritable bowel-syndrome-treatment market is experiencing a surge in patient education initiatives. Healthcare professionals in France are recognizing the importance of educating patients about IBS, its triggers, and management strategies. This focus on education is crucial, as informed patients are more likely to engage in their treatment plans and adhere to prescribed therapies. Various organizations and healthcare providers are developing resources, including workshops and informational materials, to empower patients with knowledge. This trend not only improves patient outcomes but also fosters a more collaborative relationship between patients and healthcare providers. As patients become more knowledgeable about their condition, the demand for tailored treatment options is likely to increase, further driving market growth.
Advancements in Pharmaceutical Research
Innovations in pharmaceutical research are propelling the irritable bowel-syndrome-treatment market forward in France. The development of new medications, including novel classes of drugs targeting specific IBS symptoms, has expanded treatment options for patients. For instance, the introduction of medications that focus on gut-brain interactions has shown promising results in clinical trials. This advancement is crucial, as it addresses the multifaceted nature of IBS, which often includes both gastrointestinal and psychological components. Furthermore, the French government has been supportive of research initiatives, allocating funding to enhance the understanding of IBS and its treatment. As a result, the market is witnessing a surge in new product launches, which is likely to attract more patients seeking effective solutions for their condition.
Regulatory Support for Innovative Treatments
Regulatory support for innovative treatments is a key driver in the irritable bowel-syndrome-treatment market. In France, health authorities are increasingly facilitating the approval process for new therapies aimed at IBS. This supportive regulatory environment encourages pharmaceutical companies to invest in research and development, leading to a more diverse range of treatment options for patients. The French National Agency for the Safety of Medicines and Health Products (ANSM) has streamlined procedures for clinical trials, allowing for quicker access to new medications. As a result, patients benefit from timely access to cutting-edge therapies that address their specific needs. This regulatory landscape not only enhances the market's attractiveness for investors but also ensures that patients receive the most effective treatments available.
Growing Demand for Non-Pharmacological Treatments
There is a notable shift towards non-pharmacological treatments in the irritable bowel-syndrome-treatment market. Patients in France are increasingly seeking alternative therapies, such as dietary modifications, probiotics, and psychological interventions, to manage their symptoms. This trend is driven by a desire for holistic approaches that minimize reliance on medications, which may have side effects. Research indicates that dietary changes, particularly the low-FODMAP diet, can significantly alleviate IBS symptoms for many individuals. Additionally, the integration of psychological therapies, such as cognitive-behavioral therapy, has been shown to improve patient outcomes. As awareness of these options grows, healthcare providers are adapting their treatment plans to include a broader spectrum of therapies, thereby enhancing patient satisfaction and potentially increasing market share.