Increased Regulatory Support
The medical morphine market in France benefits from a supportive regulatory environment that facilitates the approval and distribution of morphine products. Recent initiatives by the French government aim to streamline the approval process for essential medications, including opioids. This regulatory support is crucial, as it ensures that patients have timely access to necessary pain relief options. Additionally, the French Agency for the Safety of Health Products has implemented guidelines to promote the responsible use of opioids, which may enhance public trust in morphine therapies. As a result, the medical morphine market is likely to see sustained growth, driven by improved access and regulatory clarity.
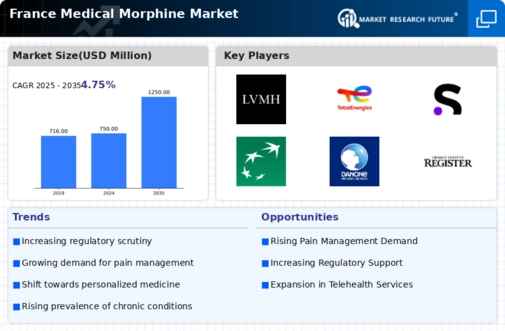
Rising Pain Management Needs
The medical morphine market in France is experiencing growth due to an increasing demand for effective pain management solutions. As the population ages, the prevalence of chronic pain conditions rises, necessitating the use of potent analgesics like morphine. According to recent data, approximately 20% of the French population suffers from chronic pain, which drives the need for medical morphine. Furthermore, healthcare providers are increasingly recognizing the importance of addressing pain as a critical component of patient care. This shift in focus towards comprehensive pain management strategies is likely to bolster the medical morphine market, as more patients seek relief from debilitating pain conditions.
Growing Awareness of Palliative Care
There is a notable increase in the recognition of palliative care's importance within the French healthcare system, which positively influences the medical morphine market. Palliative care focuses on providing relief from the symptoms and stress of serious illnesses, and morphine is a cornerstone of this approach. As healthcare professionals and patients alike become more aware of the benefits of palliative care, the demand for morphine as a key component of pain management is expected to rise. This trend is reflected in the growing number of palliative care programs across France, which may lead to an expanded role for morphine in treating patients with life-limiting conditions.
Advancements in Pharmaceutical Research
Innovations in pharmaceutical research are significantly impacting the medical morphine market in France. Ongoing studies aim to enhance the efficacy and safety profiles of morphine formulations, potentially leading to new products that cater to diverse patient needs. For instance, research into extended-release formulations may provide longer-lasting pain relief, thereby improving patient compliance. The French pharmaceutical industry has invested heavily in research and development, with expenditures reaching €5 billion in recent years. This commitment to innovation is expected to yield new morphine-based therapies, further expanding the medical morphine market and offering patients more tailored pain management options.
Economic Factors Influencing Healthcare Spending
Economic conditions in France are playing a pivotal role in shaping the medical morphine market. With healthcare spending projected to increase by 3% annually, there is a greater allocation of resources towards pain management solutions. This economic growth allows for enhanced funding for hospitals and clinics, enabling them to procure essential medications, including morphine. Furthermore, as the government prioritizes healthcare spending, patients are likely to experience improved access to pain relief options. Consequently, the medical morphine market is poised for growth, driven by favorable economic conditions that support investment in effective pain management therapies.

















Leave a Comment