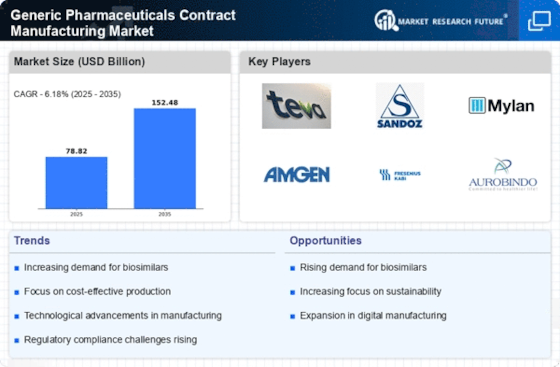
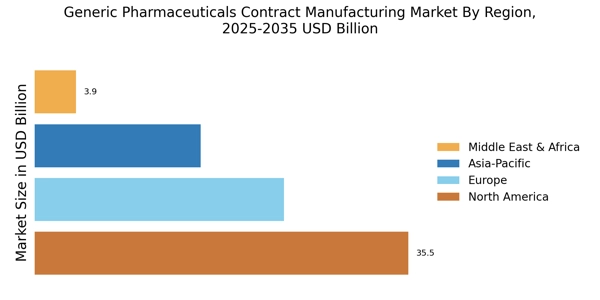
Growing Aging Population
The demographic shift towards an aging population is a significant driver for the Generic Pharmaceuticals Contract Manufacturing Market. As the global population ages, the prevalence of chronic diseases and the demand for long-term medication management increase. This demographic trend is expected to result in a higher consumption of pharmaceuticals, particularly generics, which are often more affordable alternatives to branded drugs. Data suggests that by 2030, the number of individuals aged 60 and above will surpass 1 billion, leading to an increased need for cost-effective healthcare solutions. Consequently, pharmaceutical companies are likely to outsource production to contract manufacturers to meet this rising demand efficiently. The Generic Pharmaceuticals Contract Manufacturing Market stands to benefit from this demographic trend, as it aligns with the need for sustainable and accessible healthcare solutions.
Rising Focus on Sustainability
The growing emphasis on sustainability within the pharmaceutical sector is becoming a notable driver for the Generic Pharmaceuticals Contract Manufacturing Market. As environmental concerns gain prominence, pharmaceutical companies are increasingly seeking to adopt sustainable practices in their operations. This includes the use of eco-friendly materials, waste reduction strategies, and energy-efficient manufacturing processes. Data indicates that companies implementing sustainable practices can enhance their market competitiveness and appeal to environmentally conscious consumers. Furthermore, contract manufacturers that prioritize sustainability may attract more partnerships as pharmaceutical companies look to align with suppliers that share their commitment to environmental responsibility. Thus, the Generic Pharmaceuticals Contract Manufacturing Market is likely to evolve in response to these sustainability trends, fostering innovation and collaboration in the sector.
Increasing Healthcare Expenditure
The rising healthcare expenditure across various regions appears to be a pivotal driver for the Generic Pharmaceuticals Contract Manufacturing Market. As governments and private sectors allocate more funds towards healthcare, the demand for affordable medications intensifies. This trend is particularly evident in emerging economies, where healthcare budgets are expanding to improve access to essential medicines. According to recent data, healthcare spending is projected to grow at a compound annual growth rate of approximately 5.4% over the next few years. This increase in expenditure is likely to bolster the contract manufacturing sector, as pharmaceutical companies seek to outsource production to meet the growing demand for generic drugs. Consequently, the Generic Pharmaceuticals Contract Manufacturing Market is poised for substantial growth as it aligns with the overarching goal of enhancing healthcare accessibility.
Regulatory Support for Generic Drugs
Regulatory frameworks that favor the production and distribution of generic drugs significantly influence the Generic Pharmaceuticals Contract Manufacturing Market. Governments worldwide are increasingly recognizing the importance of generics in reducing healthcare costs and improving patient access to medications. Initiatives such as expedited approval processes for generic formulations and incentives for manufacturers are becoming more common. For instance, the introduction of policies that encourage the use of generics has led to a notable increase in their market share, with generics accounting for over 90% of prescriptions in some regions. This regulatory support not only enhances the competitiveness of generic manufacturers but also stimulates the contract manufacturing sector, as pharmaceutical companies look to capitalize on favorable conditions. Thus, the Generic Pharmaceuticals Contract Manufacturing Market is likely to thrive under these supportive regulatory environments.
Expansion of Biologics and Biosimilars
The expansion of biologics and biosimilars is emerging as a crucial driver for the Generic Pharmaceuticals Contract Manufacturing Market. As the market for biologics continues to grow, the demand for biosimilars, which are essentially generic versions of biologic drugs, is also on the rise. This trend is supported by the increasing number of biologic drugs losing patent protection, creating opportunities for manufacturers to produce biosimilars. The biosimilars market is projected to reach approximately USD 100 billion by 2025, indicating a substantial growth potential. Pharmaceutical companies are likely to seek contract manufacturing services to navigate the complexities of biologics production, which often requires specialized knowledge and technology. Therefore, the Generic Pharmaceuticals Contract Manufacturing Market is expected to experience significant growth as it adapts to the evolving landscape of biologics and biosimilars.
.png)