Rising Demand for Clinical Trials
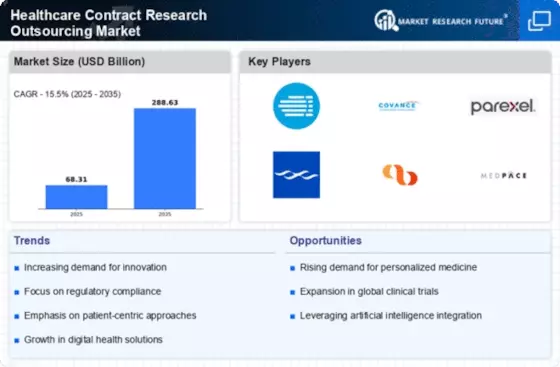
The Healthcare Contract Research Outsourcing Market is experiencing a notable increase in demand for clinical trials. This surge is primarily driven by the growing need for innovative therapies and treatments across various therapeutic areas. As pharmaceutical and biotechnology companies seek to expedite the drug development process, they are increasingly outsourcing clinical trials to specialized contract research organizations (CROs). According to recent data, the number of clinical trials initiated has risen significantly, with thousands of trials currently active worldwide. This trend indicates a robust pipeline of new drugs and therapies, which in turn fuels the growth of the Healthcare Contract Research Outsourcing Market. The ability of CROs to provide expertise and resources allows sponsors to navigate complex regulatory environments more efficiently, thereby enhancing the overall efficiency of clinical research.
Cost Efficiency and Resource Optimization
Cost efficiency remains a critical driver within the Healthcare Contract Research Outsourcing Market. Pharmaceutical companies are under constant pressure to reduce operational costs while maintaining high-quality standards in clinical research. By outsourcing to CROs, companies can leverage specialized expertise and infrastructure without the burden of maintaining in-house capabilities. This approach not only reduces costs associated with staffing and facilities but also allows for better allocation of resources towards core business functions. Recent analyses suggest that outsourcing can lead to cost savings of up to 30% in clinical trial expenditures. As a result, the trend towards outsourcing is likely to continue, further propelling the growth of the Healthcare Contract Research Outsourcing Market as companies seek to enhance their financial performance while ensuring compliance with regulatory requirements.
Increasing Focus on Personalized Medicine
The shift towards personalized medicine is emerging as a pivotal driver in the Healthcare Contract Research Outsourcing Market. As the understanding of genetic and molecular factors in disease progresses, there is a growing emphasis on developing tailored therapies that cater to individual patient needs. This paradigm shift necessitates more complex clinical trial designs, which often require specialized expertise and resources that CROs can provide. The demand for personalized medicine is reflected in the increasing number of trials focused on biomarkers and targeted therapies. Recent statistics indicate that a significant portion of new drug approvals is now associated with personalized treatment approaches. Consequently, the Healthcare Contract Research Outsourcing Market is likely to benefit from this trend, as sponsors seek to collaborate with CROs that possess the necessary capabilities to conduct innovative and patient-centric research.
Regulatory Compliance and Quality Assurance
Regulatory compliance is a paramount concern for organizations involved in clinical research, making it a significant driver in the Healthcare Contract Research Outsourcing Market. The increasing complexity of regulatory frameworks across different regions necessitates a thorough understanding of local laws and guidelines. CROs, with their specialized knowledge and experience, are well-equipped to navigate these challenges, ensuring that clinical trials adhere to stringent regulatory standards. This capability not only mitigates risks associated with non-compliance but also enhances the quality of research outcomes. As regulatory bodies continue to emphasize the importance of quality assurance in clinical trials, the demand for CROs that can provide comprehensive compliance support is expected to rise. Consequently, this trend is likely to bolster the Healthcare Contract Research Outsourcing Market as organizations prioritize regulatory adherence in their research endeavors.
Technological Advancements in Research Methodologies
Technological advancements are reshaping the landscape of the Healthcare Contract Research Outsourcing Market. The integration of innovative technologies such as artificial intelligence, machine learning, and data analytics is revolutionizing clinical trial design and execution. These technologies enable more efficient patient recruitment, real-time data monitoring, and enhanced data management, ultimately leading to faster and more reliable research outcomes. The adoption of electronic data capture systems and remote monitoring tools has also gained traction, allowing for greater flexibility and efficiency in trial management. As these technologies continue to evolve, they are likely to attract more sponsors to outsource their clinical research activities to CROs that can leverage these advancements. This trend underscores the potential for growth within the Healthcare Contract Research Outsourcing Market as organizations seek to harness the power of technology to improve research efficiency.