Regulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
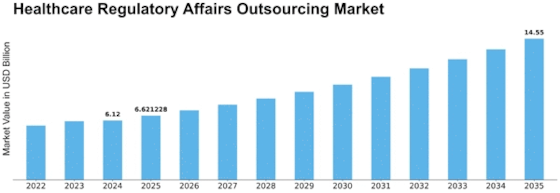
Germany Outlook (USD Billion, 2018-2032)
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies
Healthcare Regulatory Affairs Outsourcing Market by ServiceRegulatory writing And publishing
Regulatory Submission
Clinical Trial applications and services registrations
Regulatory consulting and legal representation
Others regulatory affairs
Mid size pharmaceutical companies
Large pharmaceutical companies
Biotechnology companies
Medical device companies
Food and beverage companies


















Leave a Comment