Demand for Cost-Effective Solutions
In the healthcare regulatory-affairs-outsourcing market, there is a growing demand for cost-effective solutions. Organizations are increasingly recognizing the financial benefits of outsourcing regulatory affairs, as it allows them to reduce operational costs while maintaining compliance with regulatory standards. This trend is particularly relevant in South Korea, where companies are under pressure to optimize their budgets. The outsourcing market is projected to grow by 7% as businesses seek to balance quality and cost. By leveraging external expertise, organizations can streamline their regulatory processes, ultimately leading to enhanced efficiency and reduced time to market for new products.
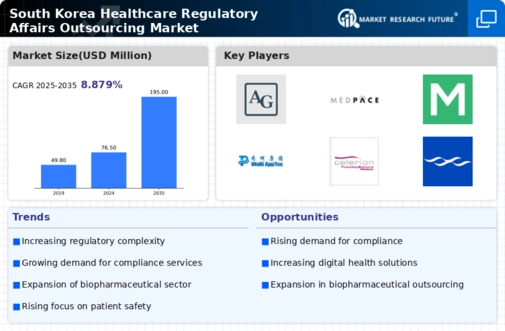
Expansion of Biopharmaceutical Sector
The biopharmaceutical sector in South Korea is witnessing rapid expansion, which significantly impacts the healthcare regulatory-affairs-outsourcing market. With the increasing number of biopharmaceutical companies entering the market, there is a heightened demand for regulatory expertise to navigate the complex approval processes. This surge in activity is expected to contribute to a market growth rate of around 12% in the outsourcing segment. Companies are increasingly outsourcing regulatory affairs to manage the intricacies of drug development and approval, thereby facilitating faster market entry for innovative therapies. This trend highlights the symbiotic relationship between the biopharmaceutical industry and the healthcare regulatory-affairs-outsourcing market.
Rising Complexity of Regulatory Frameworks
The healthcare regulatory-affairs-outsourcing market is experiencing a notable increase in the complexity of regulatory frameworks. This complexity arises from evolving laws and guidelines that govern the healthcare sector in South Korea. As regulations become more intricate, companies are compelled to seek specialized outsourcing services to navigate these challenges effectively. The demand for compliance with local and international standards has surged, leading to a projected growth rate of approximately 8% in the outsourcing sector. This trend indicates that organizations are increasingly relying on external expertise to ensure adherence to regulatory requirements, thereby driving the healthcare regulatory-affairs-outsourcing market.
Increased Investment in Healthcare Technology
The healthcare regulatory-affairs-outsourcing market is being influenced by the rising investment in healthcare technology. As South Korea continues to innovate in medical technologies, regulatory compliance becomes paramount. Companies are increasingly outsourcing regulatory affairs to ensure that their technological advancements meet the necessary regulatory standards. This trend is likely to drive market growth by approximately 9% as organizations seek to leverage external expertise in navigating the regulatory landscape. The integration of technology in healthcare processes necessitates a robust regulatory framework, further emphasizing the importance of outsourcing in maintaining compliance and fostering innovation.
Growing Focus on Patient Safety and Quality Assurance
In the healthcare regulatory-affairs-outsourcing market, there is an escalating emphasis on patient safety and quality assurance. Regulatory bodies in South Korea are intensifying their scrutiny of healthcare practices, necessitating that organizations prioritize compliance with safety standards. This focus is reflected in the increasing investments in outsourcing services that specialize in quality management systems and regulatory compliance. As a result, the market is projected to expand by 10% over the next few years, as companies seek to enhance their operational efficiency while ensuring patient safety. This trend underscores the critical role of outsourcing in maintaining high standards within the healthcare sector.