Rising Incidence of Hernias
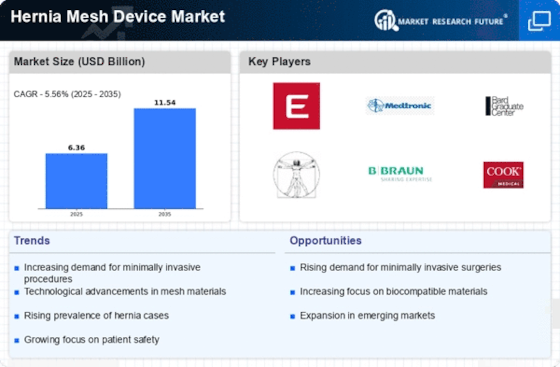
The increasing prevalence of hernias is a primary driver for the Hernia Mesh Device Market. Factors such as aging populations, sedentary lifestyles, and obesity contribute to a higher incidence of hernias. According to recent data, the incidence of inguinal hernias is estimated to be around 27 per 100,000 individuals annually. This growing patient population necessitates effective surgical interventions, thereby propelling the demand for hernia mesh devices. As more individuals seek surgical solutions, the Hernia Mesh Device Market is likely to experience substantial growth, with projections indicating a compound annual growth rate (CAGR) of approximately 5.5% over the next several years. This trend underscores the urgent need for innovative and effective hernia repair solutions.
Rising Healthcare Expenditure
The escalation of healthcare expenditure across various regions is a significant driver for the Hernia Mesh Device Market. As healthcare systems invest more in surgical technologies and patient care, the availability of advanced hernia mesh devices is likely to increase. This trend is particularly evident in developed economies, where healthcare budgets are expanding to accommodate innovative medical solutions. The World Health Organization indicates that healthcare spending is projected to rise by 5% annually in many countries, which could facilitate greater access to hernia repair surgeries. Consequently, the Hernia Mesh Device Market stands to benefit from this increased financial commitment to healthcare, as more patients gain access to necessary surgical interventions.
Regulatory Support and Approvals
Regulatory support and streamlined approval processes for hernia mesh devices are crucial factors influencing the Hernia Mesh Device Market. Regulatory bodies are increasingly recognizing the importance of efficient pathways for the approval of innovative medical devices. This trend is evident in the expedited review processes for new mesh technologies that demonstrate safety and efficacy. As regulatory frameworks evolve to support innovation, manufacturers are likely to introduce new products more rapidly, thereby enhancing competition within the market. This dynamic could lead to a wider array of options for healthcare providers and patients alike, ultimately driving growth in the Hernia Mesh Device Market as new and improved devices become available.
Increasing Awareness and Education
The growing awareness and education regarding hernia repair options are pivotal in driving the Hernia Mesh Device Market. Healthcare professionals and patients are becoming more informed about the benefits of surgical interventions, including the use of mesh devices. Educational campaigns and resources provided by medical organizations are helping to demystify the surgical process and highlight the advantages of using mesh for hernia repairs. This increased awareness is likely to lead to higher rates of diagnosis and treatment, thereby boosting the demand for hernia mesh devices. Furthermore, as patients become more proactive in seeking treatment, the Hernia Mesh Device Market may see a notable uptick in sales, contributing to a more robust market landscape.
Technological Innovations in Mesh Design
Technological advancements in the design and materials of hernia mesh devices are significantly influencing the Hernia Mesh Device Market. Innovations such as the development of lightweight, biocompatible materials and the introduction of absorbable meshes are enhancing surgical outcomes and patient satisfaction. These advancements not only improve the efficacy of hernia repairs but also reduce the risk of complications, such as chronic pain and mesh migration. The market is witnessing a shift towards more sophisticated mesh designs that cater to specific types of hernias, which could potentially increase the market size. As a result, the Hernia Mesh Device Market is expected to expand, driven by the continuous evolution of product offerings that meet the diverse needs of healthcare providers and patients.














Leave a Comment