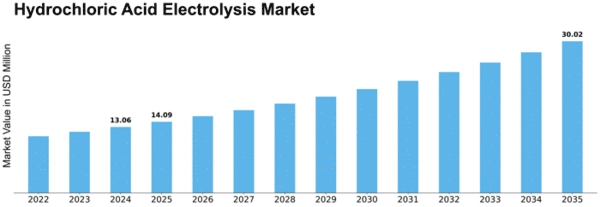
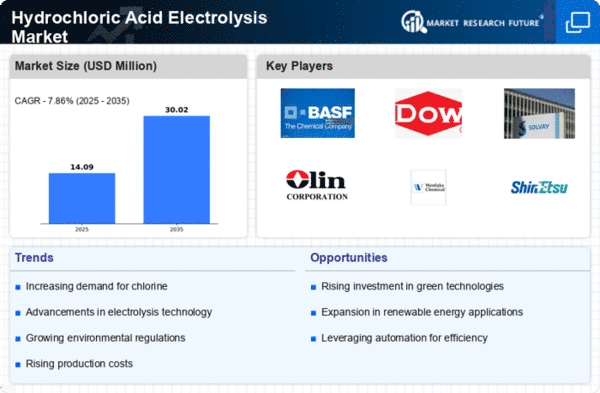
Hydrochloric Acid Electrolysis Size
Hydrochloric Acid Electrolysis Market Growth Projections and Opportunities
Industrially produced hydrochloric acid often results as a byproduct of processes like chlorination, which involves treating organic compounds with chlorine. The reaction produces hydrochloric acid (HCl) as a byproduct, and recovering chlorine from HCl is crucial in industries due to its insufficient purity or low concentration for recycling. Additionally, to adhere to strict environmental regulations, industries need to neutralize HCl before disposal.
The worldwide hydrochloric acid electrolysis market is quite dynamic and is anticipated to experience significant growth in the coming years. This growth is primarily driven by the increasing demand for liberating businesses from the uncertainties of fluctuating chlorine and hydrochloric acid prices. Additionally, the adoption of on-site HCl electrolysis technology eliminates the necessity to build new chlor-alkali plants specifically for chlorine production. However, the presence of other commercially viable technologies for chlorine production and concerns about the environmental impact of chlorine are expected to pose challenges for the market.
HCl electrolysis offers a solution for chlorine recovery from hydrogen chloride or hydrochloric acid and comes with various advantages, contributing to the sustainability of business operations. One key benefit is that it makes businesses less dependent on fluctuating chlorine and hydrochloric acid prices in the external market. As chlorine is a fundamental chemical in various industries for producing essential products, the industrial importance of recovering chlorine through HCl electrolysis is increasing over time. Moreover, this method reduces the need for building new chlor-alkali plants for chlorine production and eliminates the risks associated with transporting and storing HCl.
However, some challenges hinder the growth of the hydrochloric acid electrolysis market. Other established chlor-alkali processes for chlorine recovery and the environmental impact of chlorine, given its high reactivity and transportation risks, are notable drawbacks.
The global hydrochloric acid electrolysis market is expected to grow at a Compound Annual Growth Rate (CAGR) of 5.11% during the forecast period. In 2016, Asia-Pacific led the market with a 55.6% share, followed by North America and Europe with shares of 20.7% and 16.5%, respectively.


















Leave a Comment