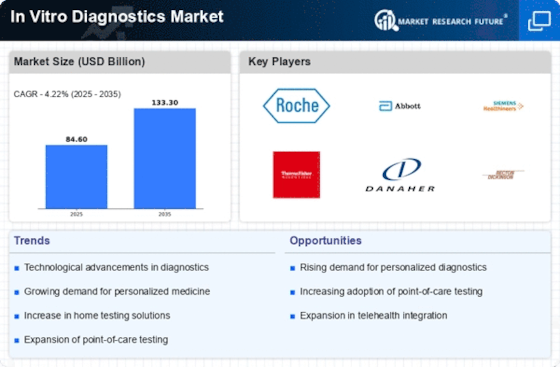
Top Industry Leaders in the In Vitro Diagnostics Market
 In Vitro Diagnostics Key Companies" width="600" height="300">*Disclaimer: List of key companies in no particular orderLatest in vitro diagnostic (IVD) Companies Update
In Vitro Diagnostics Key Companies" width="600" height="300">*Disclaimer: List of key companies in no particular orderLatest in vitro diagnostic (IVD) Companies Update
-
Sep 2023: With a focus on cancer treatment, women's health, and breathing disorders, Abbott Laboratories and mAbxience Holdings S.L., a Spanish global biotech business, have a deal to launch several biosimilars in emerging markets. The first compounds should go on sale in 2025, while others depend on how quickly clinical development and licensing can be completed. Abbott will register and sell the biosimilars in important developing nations in Southeast Asia, the Middle East, Latin America, and Africa. MAbxience will produce the biosimilars at its GMP-approved facilities in Argentina and Spain. Additionally, mAbxience will oversee seeing some of the still-in-development compounds through their clinical milestones. The partnership strengthens an existing arrangement with mAbxience, started in Latin America in 2018, and broadens Abbott's medication selection in emerging nations.
-
Oct 2023: Six new recombinant monoclonal anti-idiotypic antibodies specific to dupilumab (Dupixent) and new ipilimumab (Yervoy), evolocumab (Repatha), and secukinumab (Cosentyx) antibodies have been added to Bio-Rad Laboratories, Inc.'s portfolio of recombinant monoclonal anti-idiotypic antibodies. These pre-made antibodies can be consumed to create accurate anti-drug antibody (ADA) and pharmacokinetic (PK) assays for the drugs ipilimumab, dupilumab, evolocumab, and secukinumab, as well as their biosimilars. To become the first supplier of antibodies specific to the drug-target complex, Bio-Rad has also increased the scope of its anti-ipilimumab and anti-secukinumab product lines. This enables the detection of bound drugs.
List of in vitro diagnostic (IVD) Key companies in the market
- Abbott Laboratories,
- Bio-Rad Laboratories Inc.,
- Hoffman-LA Roche AG,
- Grifols S.A.,
- bioMerieux S.A.,
- DiaSorin S.p.A.,
- Ortho Clinical Diagnostics,
- Qiagen N.V.,
- Siemens AG,
- Becton Dickinson & Company