Focus on Cost Efficiency
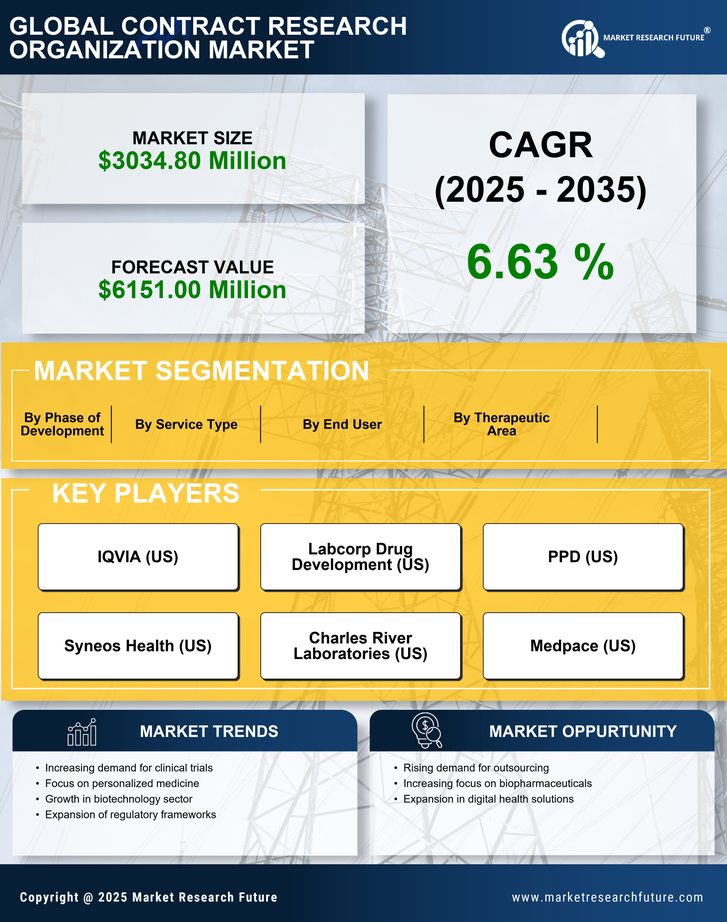
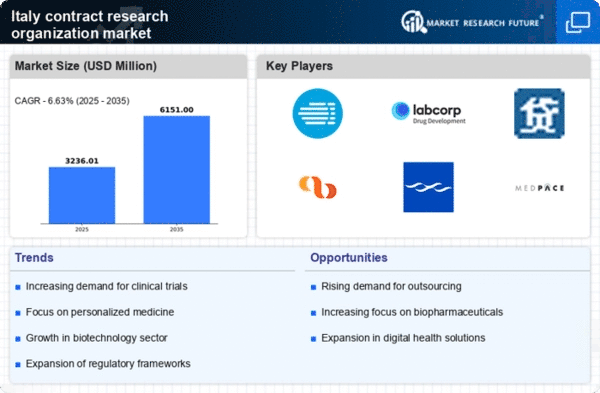
Cost efficiency remains a pivotal driver in the contract research-organization market. As pharmaceutical companies face mounting pressure to reduce operational costs, many are opting to outsource clinical trials to contract research organizations. This strategy allows them to leverage the expertise and infrastructure of these organizations while minimizing expenses. In 2025, it is projected that outsourcing could reduce clinical trial costs by up to 30%, making it an attractive option for companies looking to optimize their budgets. This trend underscores the importance of contract research organizations in providing cost-effective solutions in the competitive landscape of drug development.
Growth of Biotech Sector
The burgeoning biotechnology sector in Italy is significantly impacting the contract research-organization market. With an increasing number of biotech startups emerging, there is a heightened need for specialized research services. These startups often lack the resources to conduct extensive clinical trials independently, leading them to partner with contract research organizations. In 2025, the Italian biotech industry is expected to grow by 20%, creating a substantial market for contract research services. This growth not only provides opportunities for contract research organizations but also enhances the overall landscape of drug development in Italy.
Regulatory Support and Framework
The regulatory environment in Italy plays a crucial role in shaping the contract research-organization market. Recent initiatives by the Italian Medicines Agency (AIFA) aim to streamline the approval process for clinical trials, thereby encouraging more pharmaceutical companies to engage with contract research organizations. The introduction of more flexible regulations is expected to enhance the attractiveness of Italy as a destination for clinical research. In 2025, it is anticipated that the number of clinical trials conducted in Italy will increase by 15%, further driving the demand for contract research services. This supportive regulatory framework is likely to foster innovation and collaboration within the industry.
Rising Demand for Drug Development
The contract research-organization market in Italy is experiencing a notable increase in demand for drug development services. This trend is driven by the growing need for innovative therapies and the complexity of clinical trials. As pharmaceutical companies seek to expedite their drug development processes, they are increasingly turning to contract research organizations for expertise and resources. In 2025, the Italian pharmaceutical sector is projected to invest approximately €3 billion in research and development, which is likely to bolster the contract research-organization market. This investment reflects a broader trend towards collaboration between pharmaceutical firms and contract research organizations, enhancing efficiency and reducing time-to-market for new drugs.
Increased Collaboration with Academic Institutions
The contract research-organization market is witnessing a surge in collaboration between contract research organizations and academic institutions in Italy. These partnerships are fostering innovation and facilitating access to cutting-edge research. Academic institutions often possess unique expertise and resources that can enhance the capabilities of contract research organizations. In 2025, it is expected that collaborative projects will increase by 25%, leading to a more integrated approach to clinical research. This trend not only benefits the contract research-organization market but also contributes to the advancement of scientific knowledge and the development of new therapies.