Advancements in Biotechnology
Technological advancements in biotechnology are playing a crucial role in shaping the custom antibody market in Japan. Innovations in antibody engineering, such as the development of monoclonal antibodies and bispecific antibodies, are enhancing the specificity and efficacy of therapeutic agents. These advancements not only improve treatment outcomes but also expand the range of applications for custom antibodies in various therapeutic areas. The Japanese biotechnology sector has seen substantial investment, with funding reaching approximately $1 billion in recent years, indicating a robust environment for research and development. As these technologies continue to evolve, they are expected to drive the growth of the custom antibody market, enabling the creation of more effective and targeted therapies that meet the needs of healthcare providers and patients.
Rising Demand for Targeted Therapies
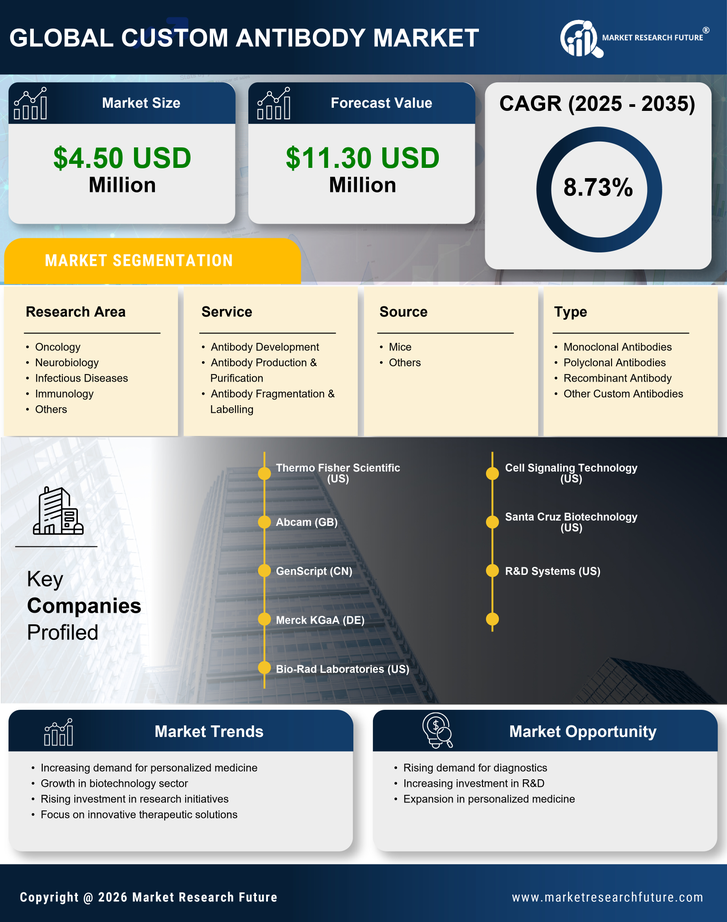
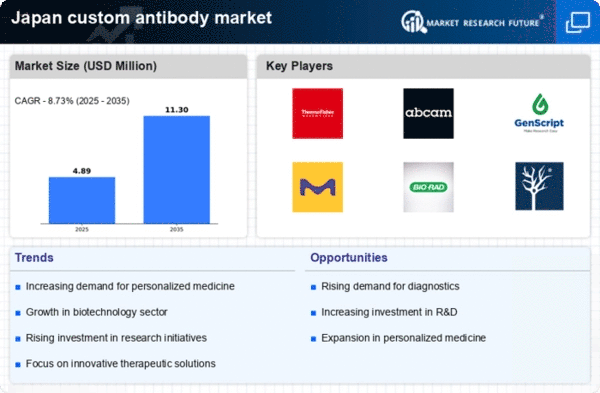
The custom antibody market in Japan is experiencing a notable surge in demand for targeted therapies. This trend is largely driven by the increasing prevalence of chronic diseases, such as cancer and autoimmune disorders, which require precise treatment modalities. As healthcare providers and patients alike seek more effective and personalized treatment options, the custom antibody market is positioned to benefit significantly. According to recent estimates, the market is projected to grow at a CAGR of approximately 8% over the next five years. This growth is indicative of a broader shift towards precision medicine, where therapies are tailored to individual patient profiles, thereby enhancing treatment efficacy and minimizing side effects. Consequently, the custom antibody market is likely to see heightened investment and innovation as stakeholders respond to this rising demand.
Regulatory Support for Biopharmaceuticals
The regulatory landscape in Japan is increasingly supportive of biopharmaceutical innovations, which is beneficial for the custom antibody market. The Pharmaceuticals and Medical Devices Agency (PMDA) has streamlined approval processes for novel therapies, including custom antibodies, thereby facilitating faster market entry. This regulatory support is crucial for companies looking to introduce innovative products, as it reduces the time and costs associated with bringing new therapies to market. Furthermore, the Japanese government has implemented various initiatives to promote biopharmaceutical research, including grants and tax incentives. As a result, the custom antibody market is likely to experience accelerated growth, with more companies entering the space and contributing to the development of cutting-edge therapies that address unmet medical needs.
Increased Focus on Diagnostics and Biomarkers
The custom antibody market in Japan is witnessing a heightened focus on diagnostics and biomarkers, which are essential for the development of personalized medicine. As healthcare professionals increasingly rely on biomarkers to guide treatment decisions, the demand for custom antibodies that can accurately detect these markers is growing. This trend is supported by advancements in diagnostic technologies, which are enabling more precise and timely identification of diseases. The market for diagnostic antibodies is projected to grow at a CAGR of around 7% over the next few years, reflecting the increasing importance of diagnostics in patient care. Consequently, the custom antibody market is likely to expand as companies develop innovative solutions that cater to the evolving needs of the healthcare landscape.
Growing Investment in Healthcare Infrastructure
Japan's commitment to enhancing its healthcare infrastructure is a significant driver for the custom antibody market. The government has allocated substantial resources to improve healthcare facilities and expand access to advanced medical technologies. This investment is expected to increase the adoption of custom antibody therapies, as healthcare providers gain access to the necessary tools and resources to implement these innovative treatments. With healthcare spending projected to reach approximately $500 billion by 2026, the custom antibody market stands to benefit from this trend. Enhanced infrastructure not only supports the delivery of advanced therapies but also fosters collaboration between research institutions and healthcare providers, further driving innovation in the custom antibody market.