Rising Healthcare Expenditure
The upward trend in healthcare expenditure in Japan is a significant driver for the hepatitis b-treatment market. As the population ages and the prevalence of chronic diseases increases, healthcare spending is projected to rise. In 2025, Japan's healthcare expenditure is expected to reach approximately ¥42 trillion, reflecting a growing commitment to improving health outcomes. This increase in funding allows for better access to hepatitis B treatments and enhances the capacity of healthcare facilities to manage chronic conditions. Furthermore, the expansion of health insurance coverage for hepatitis B treatments ensures that more patients can receive timely and effective care, thereby stimulating demand within the market.
Government Initiatives and Funding
Government initiatives aimed at combating hepatitis B are instrumental in driving the hepatitis b-treatment market. The Japanese government has implemented various policies to enhance screening, prevention, and treatment of hepatitis B. Increased funding for public health programs has facilitated access to antiviral therapies, thereby improving treatment rates. For example, the introduction of subsidized treatment options has made therapies more affordable for patients, which is crucial in a country where healthcare costs can be a barrier. Additionally, the government's commitment to eliminating hepatitis B as a public health threat by 2030 aligns with the objectives of the hepatitis b-treatment market, fostering an environment conducive to growth and innovation.
Increasing Prevalence of Hepatitis B
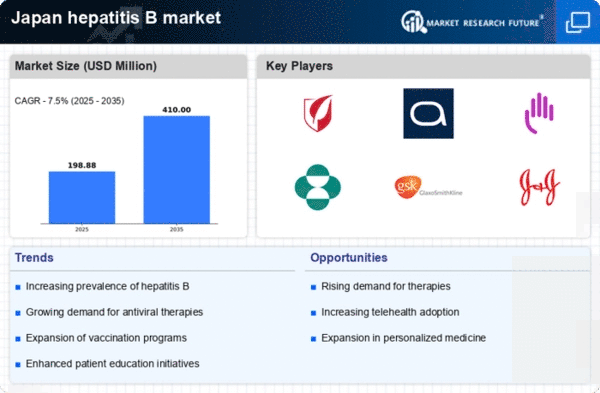
The rising prevalence of hepatitis B in Japan is a critical driver for the hepatitis b-treatment market. Recent estimates indicate that approximately 1.5 million individuals are living with chronic hepatitis B in the country. This growing patient population necessitates enhanced treatment options and healthcare services. The increasing incidence of hepatitis B infections, particularly among high-risk groups, underscores the urgent need for effective management strategies. As awareness of the disease expands, healthcare providers are likely to prioritize hepatitis B treatment, thereby stimulating market growth. Furthermore, the Japanese government has initiated various public health campaigns aimed at reducing the burden of hepatitis B, which may further contribute to the demand for treatment solutions in the market.
Growing Focus on Preventive Healthcare
The shift towards preventive healthcare in Japan is influencing the hepatitis b-treatment market. There is an increasing recognition of the importance of early detection and intervention in managing hepatitis B. Preventive measures, such as vaccination programs and regular screening, are being emphasized to reduce the incidence of new infections. The Japanese government has launched initiatives to promote hepatitis B vaccinations, particularly among high-risk populations. This proactive approach not only aims to decrease the number of new cases but also encourages individuals to seek treatment earlier, thereby driving demand for existing therapies. The focus on prevention is likely to create a more informed patient population, which may further enhance the market for hepatitis B treatments.
Technological Innovations in Treatment
Technological advancements play a pivotal role in shaping the hepatitis b-treatment market. Innovations in drug development, such as the introduction of direct-acting antivirals, have significantly improved treatment outcomes for patients. In Japan, the approval of new therapies has led to enhanced efficacy and safety profiles, which are crucial for patient adherence. The market has witnessed a surge in research and development activities, with pharmaceutical companies investing heavily in novel treatment modalities. For instance, the introduction of combination therapies has shown promising results in clinical trials, potentially leading to better management of hepatitis B. These technological innovations not only improve patient outcomes but also drive competition among manufacturers, thereby influencing pricing strategies and market dynamics.
















Leave a Comment