Growing Awareness and Education
There is a notable increase in awareness and education regarding tracheostomy procedures and products in Japan. Healthcare professionals are receiving enhanced training on the management of tracheostomy patients, which is crucial for improving patient outcomes. This educational push is likely to lead to a higher adoption rate of tracheostomy products, as clinicians become more knowledgeable about their benefits and applications. Furthermore, patient education initiatives are also gaining traction, empowering individuals and their families to understand the importance of proper tracheostomy care. As awareness levels rise, the tracheostomy products market is expected to expand, driven by informed decision-making among both healthcare providers and patients.
Increased Healthcare Expenditure
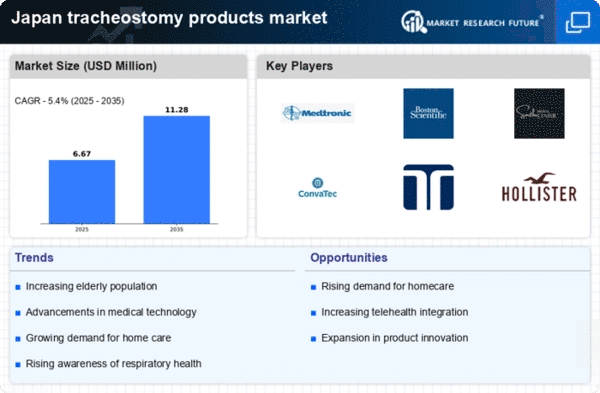
Japan's healthcare expenditure has been on the rise, which significantly impacts the tracheostomy products market. The government allocates a substantial portion of its budget to healthcare, with spending reaching approximately 10.9% of GDP. This financial commitment facilitates the procurement of advanced medical devices, including tracheostomy products. Hospitals and healthcare facilities are increasingly investing in high-quality equipment to enhance patient care, which is likely to drive market growth. Additionally, the introduction of innovative products that improve patient comfort and safety is expected to attract more investments. As healthcare providers prioritize effective treatment options, the tracheostomy products market is poised to benefit from this upward trend in healthcare spending.
Supportive Regulatory Environment
The regulatory environment in Japan is becoming increasingly supportive of the tracheostomy products market. The Pharmaceuticals and Medical Devices Agency (PMDA) has streamlined the approval process for medical devices, facilitating quicker access to innovative tracheostomy products. This regulatory support encourages manufacturers to invest in research and development, leading to the introduction of new and improved products. Moreover, the emphasis on patient safety and efficacy in regulatory guidelines ensures that only high-quality devices reach the market. As a result, the tracheostomy products market is expected to thrive, bolstered by a favorable regulatory landscape that promotes innovation and enhances patient care.
Rising Incidence of Respiratory Disorders
The increasing prevalence of respiratory disorders in Japan is a crucial driver for the tracheostomy products market. Conditions such as chronic obstructive pulmonary disease (COPD) and asthma are becoming more common, leading to a higher demand for tracheostomy interventions. According to recent health statistics, approximately 3.6 million individuals in Japan suffer from COPD, which necessitates the use of tracheostomy products for effective management. This trend indicates a growing need for specialized medical devices, thereby propelling the market forward. Furthermore, the aging population is more susceptible to respiratory issues, further amplifying the demand for tracheostomy products. As healthcare providers seek to improve patient outcomes, the market is likely to experience sustained growth driven by these health challenges.
Technological Innovations in Medical Devices
Technological innovations are transforming the landscape of the tracheostomy products market. The introduction of advanced materials and designs enhances the functionality and safety of tracheostomy devices. For instance, the development of cuffed tracheostomy tubes with improved sealing capabilities reduces the risk of aspiration and enhances patient comfort. Additionally, smart tracheostomy devices equipped with monitoring features are emerging, allowing for real-time assessment of patient conditions. These innovations not only improve clinical outcomes but also attract healthcare providers seeking to implement cutting-edge solutions. As technology continues to evolve, the tracheostomy products market is likely to witness significant growth driven by these advancements.