Rising Awareness and Advocacy
The growing awareness and advocacy surrounding Juvenile Idiopathic Arthritis are crucial drivers for the Juvenile Idiopathic Arthritis Therapeutic Market. Organizations dedicated to educating the public and healthcare professionals about JIA are playing a significant role in increasing diagnosis rates and treatment accessibility. Campaigns aimed at raising awareness have led to improved understanding of the disease, which is essential for early intervention. As more families become informed about JIA, the demand for effective therapies is likely to rise. This heightened awareness not only benefits patients but also encourages pharmaceutical companies to invest in the development of new treatments, thereby fostering growth within the Juvenile Idiopathic Arthritis Therapeutic Market.
Advancements in Biologic Therapies
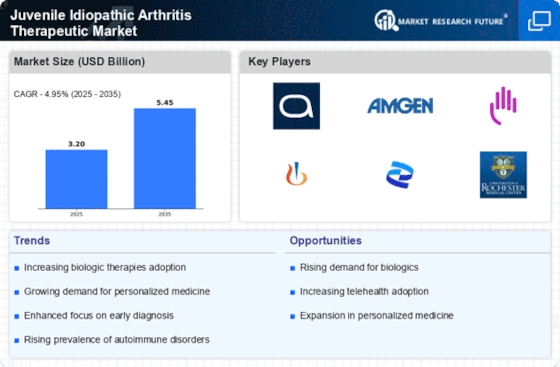
The development of biologic therapies represents a transformative shift in the Juvenile Idiopathic Arthritis Therapeutic Market. These therapies, which target specific components of the immune system, have shown promising results in managing JIA symptoms and improving quality of life for affected children. The introduction of agents such as tumor necrosis factor (TNF) inhibitors has expanded treatment options, leading to increased adoption among healthcare providers. Market data indicates that the biologics segment is expected to witness substantial growth, potentially reaching a valuation of several billion dollars in the coming years. This trend underscores the importance of continued innovation and research in the Juvenile Idiopathic Arthritis Therapeutic Market.
Integration of Digital Health Solutions
The integration of digital health solutions into the management of Juvenile Idiopathic Arthritis is emerging as a key driver for the Juvenile Idiopathic Arthritis Therapeutic Market. Digital tools, such as mobile applications and telemedicine platforms, facilitate better patient engagement and adherence to treatment regimens. These technologies enable healthcare providers to monitor patient progress remotely, which can lead to timely interventions and improved outcomes. Market analysis indicates that the adoption of digital health solutions is likely to increase, as both patients and providers recognize the benefits of enhanced communication and data sharing. This trend not only supports better management of JIA but also contributes to the overall growth of the Juvenile Idiopathic Arthritis Therapeutic Market.
Regulatory Support for Innovative Treatments
Regulatory bodies are increasingly supportive of innovative treatments for Juvenile Idiopathic Arthritis, which serves as a significant driver for the Juvenile Idiopathic Arthritis Therapeutic Market. Initiatives aimed at expediting the approval process for new therapies, particularly those that demonstrate substantial clinical benefits, are becoming more common. This regulatory environment encourages pharmaceutical companies to invest in research and development, knowing that their innovations may reach the market more swiftly. The potential for faster access to novel therapies not only benefits patients but also enhances competition within the Juvenile Idiopathic Arthritis Therapeutic Market, ultimately leading to a broader array of treatment options.
Increasing Prevalence of Juvenile Idiopathic Arthritis
The rising incidence of Juvenile Idiopathic Arthritis (JIA) is a pivotal driver for the Juvenile Idiopathic Arthritis Therapeutic Market. Recent estimates suggest that JIA affects approximately 1 in 1,000 children, leading to a growing demand for effective therapeutic options. As awareness of this condition increases, more children are being diagnosed, which in turn propels the need for innovative treatments. The increasing prevalence is likely to stimulate market growth as healthcare providers seek to address the needs of this expanding patient population. Furthermore, the long-term implications of untreated JIA can lead to significant healthcare costs, thereby emphasizing the necessity for effective management strategies within the Juvenile Idiopathic Arthritis Therapeutic Market.