Market Trends
Key Emerging Trends in the Leuprolide Acetate Market
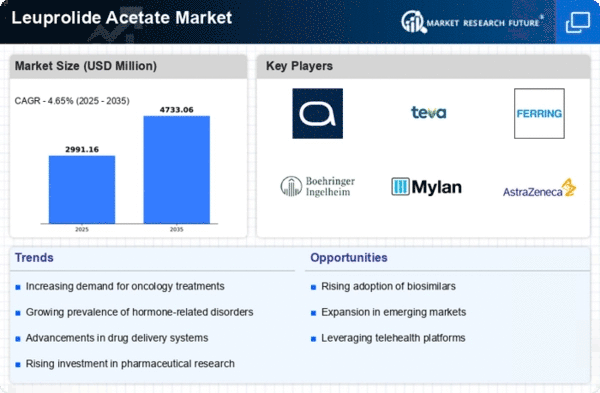
The Leuprolide Acetate market is witnessing notable trends that are shaping its landscape and influencing the overall pharmaceutical industry. Leuprolide Acetate, a synthetic gonadotropin-releasing hormone (GnRH) analog, is primarily used in the treatment of various hormonal disorders such as prostate cancer, endometriosis, and certain types of breast cancer. One prominent trend in the market is the increasing prevalence of hormone-related disorders, particularly prostate cancer. The rising incidence of prostate cancer globally has led to a growing demand for Leuprolide Acetate, making it a key player in the oncology segment.
Moreover, there is a noticeable shift towards the development of sustained-release formulations of Leuprolide Acetate. This trend is driven by the need for prolonged therapeutic effects and enhanced patient compliance. Sustained-release formulations offer the advantage of reducing the frequency of administration, improving patient adherence to treatment regimens, and ultimately enhancing the overall therapeutic outcomes. Pharmaceutical companies are investing in research and development to create innovative formulations that provide sustained and controlled release of the drug, catering to the evolving needs of both healthcare professionals and patients.
Another significant trend is the geographic expansion of market players and the increasing accessibility of Leuprolide Acetate across different regions. The globalization of pharmaceutical companies has led to improved distribution networks and broader market reach. This expansion is not only beneficial for the companies but also for patients in regions where access to advanced healthcare treatments might have been limited in the past. The broader availability of Leuprolide Acetate contributes to better patient outcomes and facilitates the standardization of treatment protocols on a global scale.
Additionally, the market is witnessing a surge in strategic collaborations and partnerships among pharmaceutical companies. These collaborations aim to leverage the strengths of each partner in terms of research, development, manufacturing, and marketing. By pooling resources and expertise, companies can expedite the drug development process, reduce costs, and bring new and improved formulations of Leuprolide Acetate to the market more efficiently. This trend not only fosters innovation but also ensures a competitive edge for companies in an ever-evolving pharmaceutical landscape.
On the regulatory front, there is an increased focus on ensuring the safety and efficacy of Leuprolide Acetate formulations. Regulatory bodies worldwide are imposing stringent guidelines and requirements for the approval of new drug formulations. This trend is a response to the growing awareness of the importance of drug safety and the need to protect patient health. Companies operating in the Leuprolide Acetate market are adapting to these regulatory changes by investing in robust clinical trials, quality assurance measures, and compliance with international standards.


















Leave a Comment