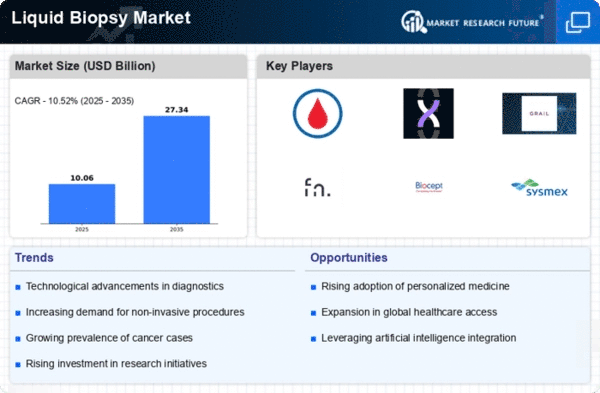
Market Trends
Key Emerging Trends in the Liquid Biopsy Market
Liquid biopsies present a revolutionary approach to medical diagnostics by sidestepping the need for painful tissue extractions, thereby alleviating patient discomfort associated with the procedure. The primary sources of liquid samples used in this non-invasive method are blood and urine, making the collection process relatively painless. Although some liquid biopsy samples involve more invasive procedures, like spinal fluid extraction, the overall reduction in pain compared to traditional tissue biopsies is a significant advantage, easing patient apprehension. Moreover, liquid biopsy offers a safe and effective alternative for individuals who may be deemed unsuitable candidates for tissue biopsies due to associated risks, such as lung cancer patients with tumors located perilously close to the heart. This reduction in pain and associated risks is a crucial driver in the market, especially for patients willing to invest a premium amount to enhance their quality of life. Traditional tissue biopsies encounter challenges stemming from a low detection rate due to the heterogeneous nature of tissues. Conducting consecutive biopsies to capture the spatial and temporal heterogeneity of tumors is not only expensive but also logistically challenging. In contrast, liquid biopsy involves the detection of free circulating DNA fragments, which are not secreted by healthy cells. Recent technological breakthroughs, such as circulating tumor DNA (ctDNA) genome mapping, and the development of biomarkers with increased sensitivity, have enabled real-time profiling and monitoring of diseases, particularly cancer. This allows for the early detection of minimal residual disease, significantly improving survival rates in life-threatening cases. Additionally, liquid biopsy has demonstrated the ability to cut down result turnaround times. Traditional tissue biopsy procedures typically take an average of 30 days to yield results, whereas liquid biopsy delivers results in approximately 12 days. With the ongoing trend of increasing automation in liquid biopsy processes, this timeframe is expected to further decrease.
The advantages offered by liquid biopsy in terms of reduced pain, lowered risks, and enhanced diagnostic efficiency have positioned it as a transformative technology in the medical field. The ability to detect diseases earlier and more accurately not only contributes to improved patient outcomes but also streamlines the diagnostic process. As automation continues to play a more significant role in liquid biopsy procedures, the technology is likely to become even more accessible and efficient, further solidifying its place as a game-changer in the realm of medical diagnostics.
















Leave a Comment