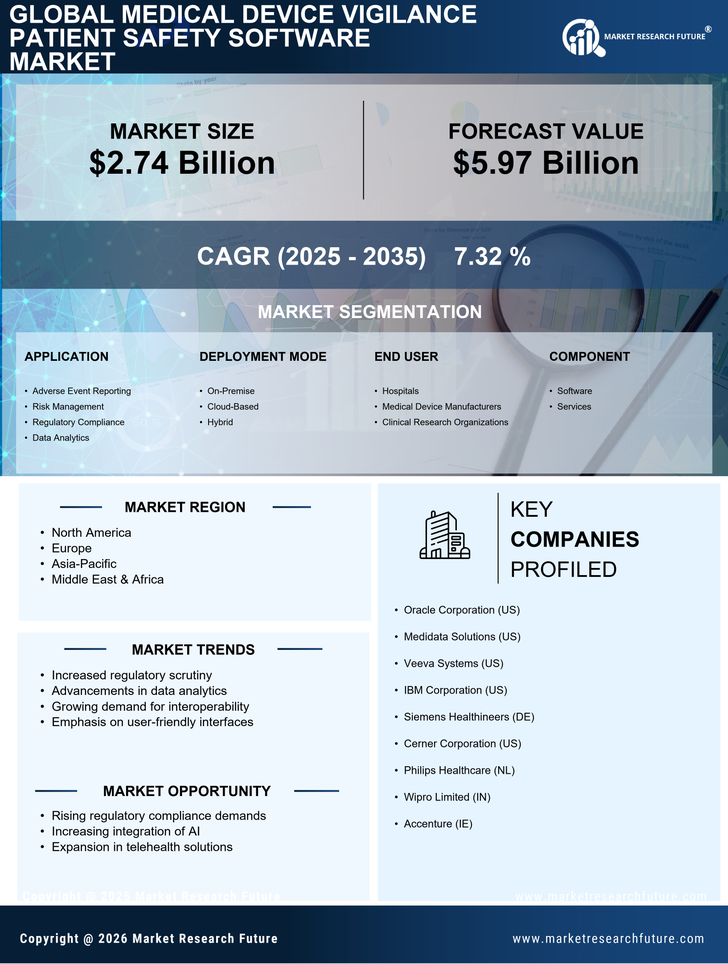
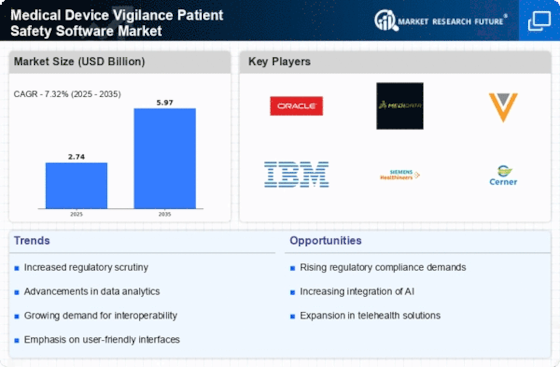
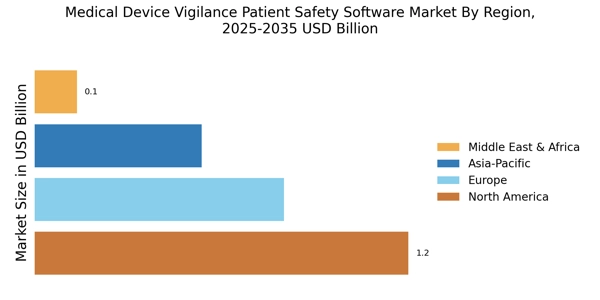
Focus on Patient-Centric Solutions
The shift towards patient-centric solutions is profoundly influencing the Medical Device Vigilance Patient Safety Software Market. Healthcare providers are increasingly recognizing the importance of patient feedback and experiences in improving device safety and efficacy. Software that incorporates patient-reported outcomes and real-time feedback mechanisms is becoming essential. This trend is supported by data indicating that patient engagement can lead to a 30% reduction in adverse events. As a result, companies are investing in software that not only monitors device performance but also actively involves patients in the safety process. This dual focus on technology and patient engagement is likely to drive market growth and innovation.
Rising Incidence of Adverse Events
The rising incidence of adverse events associated with medical devices is a significant driver for the Medical Device Vigilance Patient Safety Software Market. As the number of medical devices in use continues to grow, so does the potential for complications and safety issues. Reports indicate that adverse events have increased by approximately 15% in recent years, prompting healthcare organizations to seek more effective vigilance solutions. This trend underscores the necessity for comprehensive software that can track, analyze, and report adverse events efficiently. Consequently, the demand for advanced vigilance software is expected to rise, as organizations strive to enhance patient safety and minimize risks associated with medical devices.
Regulatory Compliance and Reporting
Regulatory compliance remains a critical driver in the Medical Device Vigilance Patient Safety Software Market. With stringent regulations imposed by health authorities, manufacturers are compelled to adopt software solutions that facilitate accurate reporting and compliance with safety standards. The increasing complexity of regulatory requirements necessitates robust software that can streamline the reporting process, ensuring timely submission of adverse event data. As of 2025, it is estimated that over 70% of medical device companies will prioritize compliance-driven software investments, reflecting a growing recognition of the importance of maintaining regulatory standards. This focus on compliance not only mitigates legal risks but also enhances the overall safety of medical devices.
Integration of Advanced Technologies
The Medical Device Vigilance Patient Safety Software Market is experiencing a notable shift towards the integration of advanced technologies such as artificial intelligence and machine learning. These technologies enhance data analysis capabilities, allowing for more accurate detection of adverse events and trends in patient safety. As a result, healthcare providers can respond more swiftly to potential risks, thereby improving patient outcomes. The market is projected to grow at a compound annual growth rate (CAGR) of approximately 12% over the next five years, driven by the increasing demand for sophisticated software solutions that can manage vast amounts of data efficiently. This trend indicates a significant opportunity for software developers to innovate and create more effective vigilance systems.
Increased Investment in Healthcare IT
Increased investment in healthcare IT infrastructure is propelling the Medical Device Vigilance Patient Safety Software Market forward. As healthcare organizations allocate more resources to technology, the demand for sophisticated software solutions that ensure patient safety is on the rise. It is estimated that healthcare IT spending will reach over $200 billion by 2026, with a significant portion directed towards patient safety software. This investment trend indicates a growing recognition of the role that technology plays in improving healthcare outcomes. Enhanced IT infrastructure not only supports the implementation of vigilance software but also facilitates better data sharing and collaboration among healthcare providers, ultimately leading to improved patient safety.