Growth of Personalized Medicine
The growth of personalized medicine is reshaping the landscape of the Molecular Quality Control Market. As healthcare shifts towards tailored treatment approaches, the demand for molecular testing that supports personalized therapies is increasing. This trend is driving the need for stringent quality control measures to ensure the accuracy of tests that guide treatment decisions. The molecular diagnostics market, which is closely linked to personalized medicine, is projected to witness substantial growth, with estimates indicating a market size of over USD 20 billion by 2025. Consequently, the Molecular Quality Control Market is likely to see increased investments in quality assurance processes that align with the principles of personalized medicine.
Rising Demand for Accurate Diagnostics
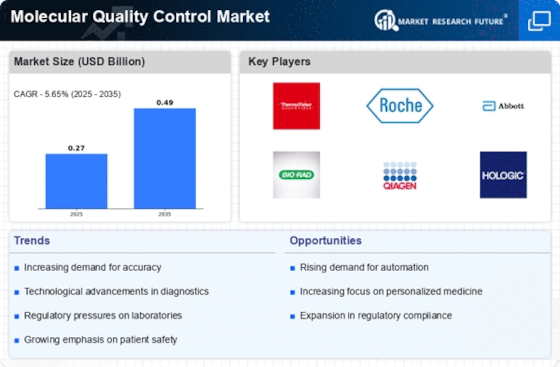
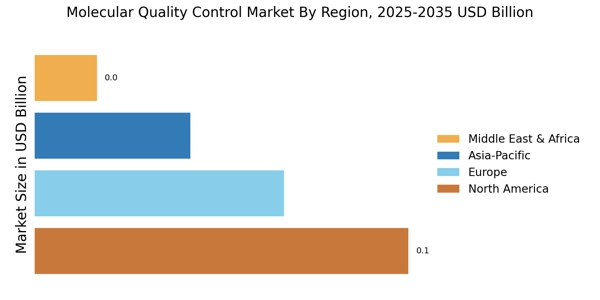
The increasing demand for accurate and reliable diagnostic tests is a primary driver of the Molecular Quality Control Market. As healthcare providers strive to enhance patient outcomes, the need for precise testing methodologies has surged. This trend is reflected in the projected growth of the molecular diagnostics market, which is expected to reach approximately USD 11 billion by 2026. The emphasis on early disease detection and personalized medicine further propels the demand for molecular quality control solutions, ensuring that diagnostic tests yield consistent and trustworthy results. Consequently, stakeholders in the Molecular Quality Control Market are likely to invest in advanced quality control measures to meet these evolving demands.
Increased Focus on Regulatory Compliance
The heightened focus on regulatory compliance is a significant driver of the Molecular Quality Control Market. Regulatory bodies are imposing stricter guidelines to ensure the safety and efficacy of diagnostic tests. This trend necessitates robust quality control measures to meet compliance requirements. For instance, the FDA has established rigorous standards for molecular diagnostic tests, compelling manufacturers to adopt comprehensive quality control protocols. As a result, the market for molecular quality control solutions is expected to expand, with stakeholders investing in systems that facilitate adherence to these regulations. The emphasis on compliance not only enhances product reliability but also fosters consumer trust in molecular diagnostics.
Technological Innovations in Quality Control
Technological advancements play a crucial role in shaping the Molecular Quality Control Market. Innovations such as automation, artificial intelligence, and real-time monitoring systems are enhancing the efficiency and accuracy of quality control processes. For instance, the integration of AI algorithms in quality control systems can significantly reduce human error and improve data analysis. The market for automated quality control solutions is anticipated to grow, with estimates suggesting a compound annual growth rate of over 10% in the coming years. These technological innovations not only streamline operations but also ensure compliance with stringent regulatory standards, thereby reinforcing the importance of quality control in molecular diagnostics.
Expansion of Research and Development Activities
The expansion of research and development activities in the field of molecular diagnostics is a key driver of the Molecular Quality Control Market. As researchers explore new biomarkers and diagnostic techniques, the need for rigorous quality control measures becomes paramount. This trend is evidenced by the increasing number of clinical trials and studies focused on molecular diagnostics, which require stringent quality assurance protocols to ensure the validity of results. The investment in R&D is expected to rise, with projections indicating a growth rate of approximately 8% annually in the coming years. This focus on innovation and quality control is likely to enhance the overall reliability of molecular diagnostics, thereby benefiting the Molecular Quality Control Market.
















Leave a Comment