Expansion of Biopharmaceutical Sector
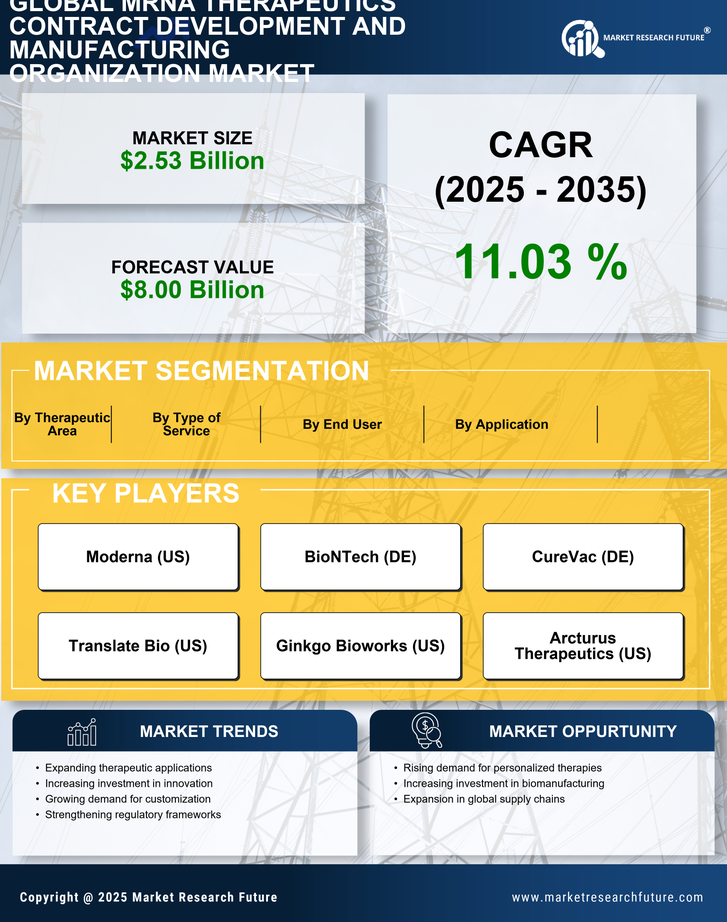
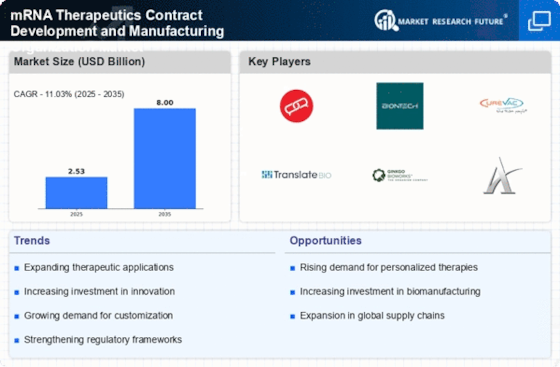
The mRNA Therapeutics Contract Development and Manufacturing Organization Market is benefiting from the overall expansion of the biopharmaceutical sector. As biopharmaceutical companies increasingly turn to mRNA technology for drug development, the demand for specialized manufacturing services is on the rise. This expansion is driven by the need for innovative therapies that address unmet medical needs across various therapeutic areas. The biopharmaceutical sector is projected to grow at a robust rate, with mRNA therapeutics playing a crucial role in this evolution. Consequently, contract development and manufacturing organizations are likely to see increased demand for their services, as they provide the necessary expertise and infrastructure to support the development of these cutting-edge therapies.
Regulatory Support for mRNA Innovations
The mRNA Therapeutics Contract Development and Manufacturing Organization Market is experiencing favorable regulatory support, which is crucial for the advancement of mRNA technologies. Regulatory agencies are increasingly recognizing the potential of mRNA therapeutics and are adapting their frameworks to facilitate faster approvals and streamlined processes. This supportive environment encourages innovation and investment in mRNA research and development. As regulatory pathways become more defined, contract development and manufacturing organizations are better equipped to navigate the complexities of compliance, thereby enhancing their value proposition in the market. The ongoing collaboration between industry stakeholders and regulatory bodies is likely to foster a more conducive landscape for mRNA therapeutics, ultimately benefiting the entire market.
Rising Demand for Personalized Medicine
The mRNA Therapeutics Contract Development and Manufacturing Organization Market is experiencing a notable increase in demand for personalized medicine. This trend is driven by advancements in genomics and biotechnology, which enable the development of tailored therapies that cater to individual patient profiles. As healthcare systems increasingly prioritize personalized treatment approaches, the need for specialized mRNA therapeutics is expected to grow. According to recent estimates, the market for personalized medicine is projected to reach substantial figures, indicating a robust growth trajectory. This shift towards individualized therapies necessitates the expertise of contract development and manufacturing organizations, which are well-positioned to support the development and production of these innovative mRNA-based treatments.
Technological Advancements in mRNA Production
Technological innovations are playing a pivotal role in shaping the mRNA Therapeutics Contract Development and Manufacturing Organization Market. Recent advancements in mRNA synthesis, purification, and formulation technologies have significantly enhanced the efficiency and scalability of mRNA production. These improvements not only reduce production costs but also increase the speed at which new therapeutics can be brought to market. For instance, the implementation of automated systems and high-throughput platforms has streamlined the manufacturing process, allowing organizations to meet the growing demand for mRNA therapeutics. As the industry continues to evolve, these technological advancements are likely to attract further investment and collaboration, thereby bolstering the overall market landscape.
Growing Focus on Infectious Disease Management
The mRNA Therapeutics Contract Development and Manufacturing Organization Market is witnessing a heightened focus on infectious disease management. This trend is largely influenced by the increasing prevalence of infectious diseases and the urgent need for effective vaccines and therapeutics. mRNA technology has emerged as a promising solution, offering rapid development timelines and the potential for broad-spectrum efficacy. As governments and health organizations allocate more resources towards combating infectious diseases, the demand for mRNA-based solutions is expected to rise. Market data suggests that investments in mRNA vaccine development are projected to reach significant levels, further underscoring the importance of contract development and manufacturing organizations in facilitating this growth.