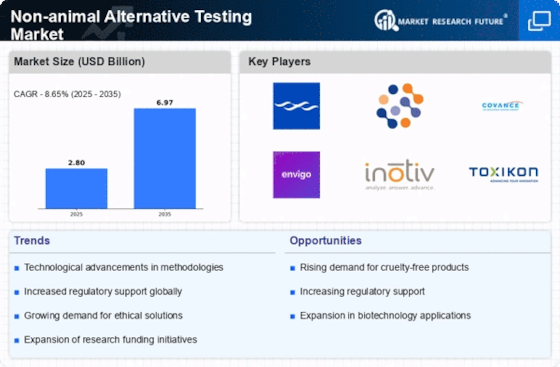
The Non-animal Alternative Testing Market has witnessed significant expansion over the past few years, primarily due to the increasing demand for humane and ethical testing methodologies. This market is characterized by innovation and technological advancements that serve as alternatives to traditional animal testing.
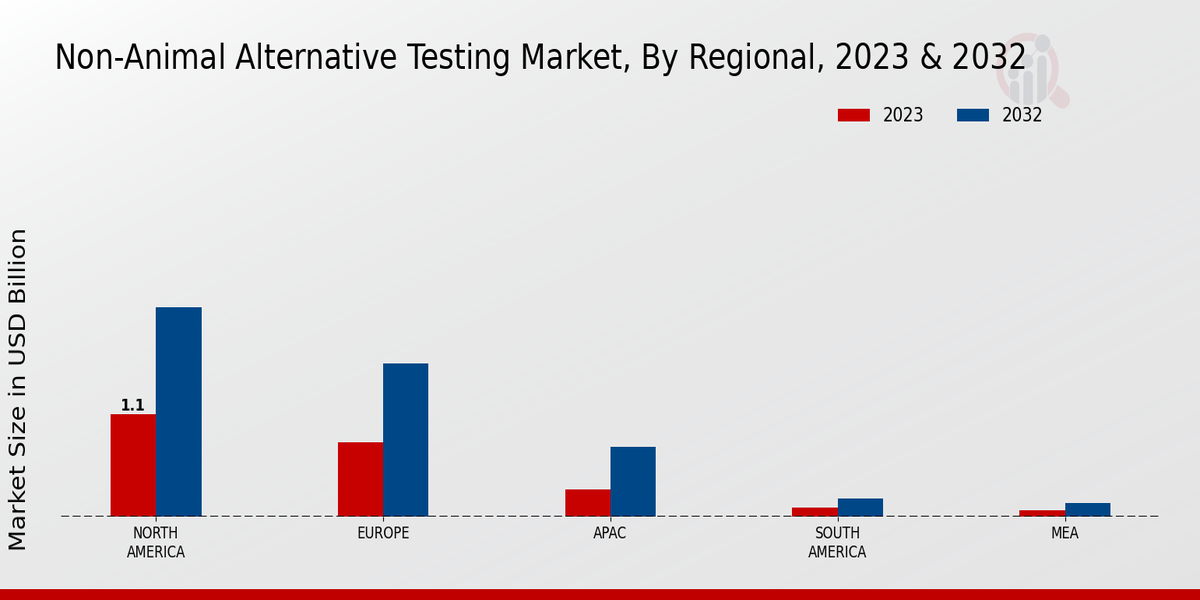
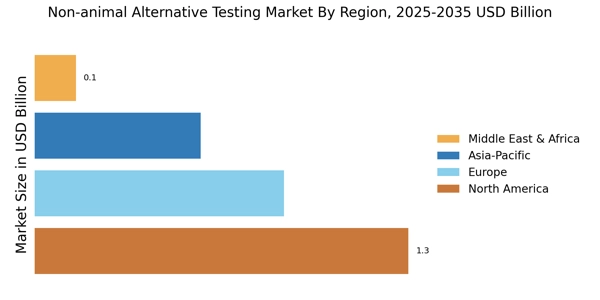
With stringent regulations and policies being enforced globally to curb animal testing, companies are investing heavily in developing non-animal-based testing methods. The competitive landscape is becoming increasingly dynamic, with a growing number of players entering the market, creating a variety of options for stakeholders in various sectors, including pharmaceuticals packaging, cosmetics, and chemicals.
Key trends driving competition include increased collaboration between industry players and research institutions, along with advancements in technologies such as in vitro testing, computer modeling, and organ-on-chip systems.
As public awareness of animal welfare issues rises, companies in the market are also aligning their strategies with sustainable practices to enhance their corporate social responsibility profiles.
Mimicry Therapeutics has established itself as a prominent player in the Non-animal Alternative Testing Market by leveraging several strengths that differentiate it from competitors. The company is recognized for its innovative approach to utilizing advanced computational models that mimic human biology, providing accurate and reliable data without the need for animal subjects.
This strong focus on technology enables Mimicry Therapeutics to offer solutions that streamline the drug development process, making it more efficient and less time-consuming for clients. Additionally, the company boasts a talented research and development team that continually seeks to enhance its testing methodologies.
The firm's dedication to quality and scientific rigor has fostered trust among its clients, allowing for a robust market presence. Furthermore, Mimicry Therapeutics actively engages in partnerships with regulatory agencies and industry associations to promote the adoption of non-animal testing methods, further bolstering its reputation and influence in the market.
Charles River Laboratories positions itself as a key contender in the Non-animal Alternative Testing Market by capitalizing on its extensive expertise in preclinical testing and research services. The company is known for its commitment to innovation and the development of alternative testing models that meet regulatory standards while minimizing the use of animals.
Charles River Laboratories has made significant investments in technological advancements, enabling it to deliver high-quality testing solutions that are both effective and ethically aligned. Their strong track record in collaborating with pharmaceutical and biotechnology firms has established a solid foundation, allowing them to integrate non-animal testing into existing workflows seamlessly.
The company's global reach and established relationships with regulatory bodies enable it to adapt quickly to changing market dynamics, making it a trusted partner for organizations seeking to transition to humane testing practices.
By maintaining a focus on scientific excellence and customer satisfaction, Charles River Laboratories continues to play a pivotal role in shaping the future of alternative testing methodologies in the market.
















Leave a Comment