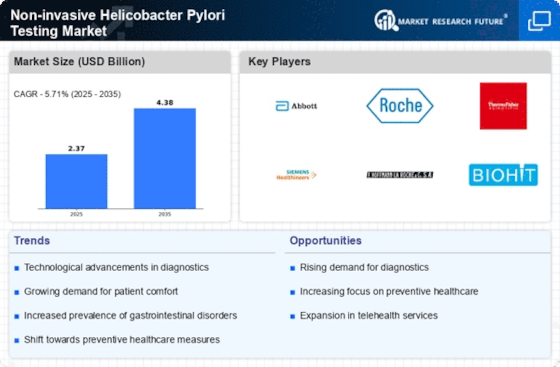
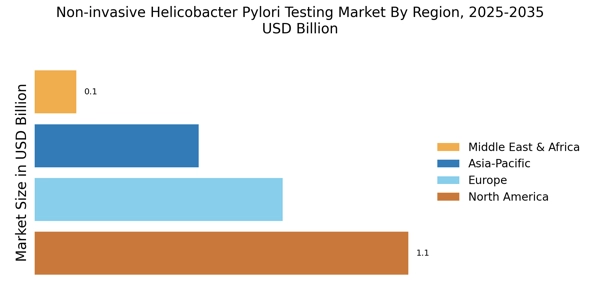
Regulatory Support and Guidelines
Regulatory support and the establishment of guidelines for Helicobacter Pylori testing are crucial factors propelling the Non-invasive Helicobacter Pylori Testing Market. Health authorities are increasingly recognizing the importance of early detection and management of Helicobacter Pylori infections, leading to the development of standardized testing protocols. These guidelines encourage healthcare providers to adopt non-invasive testing methods, which are often preferred by patients due to their convenience and safety. As regulatory bodies continue to advocate for improved testing practices, the market is likely to see enhanced adoption rates of non-invasive testing solutions. This regulatory backing not only boosts confidence among healthcare providers but also promotes patient compliance, ultimately contributing to the growth of the Non-invasive Helicobacter Pylori Testing Market.
Expansion of Healthcare Infrastructure
The expansion of healthcare infrastructure is a pivotal driver for the Non-invasive Helicobacter Pylori Testing Market. As healthcare systems evolve and improve, access to diagnostic testing becomes more widespread, particularly in underserved regions. This expansion includes the establishment of new clinics and diagnostic centers equipped with advanced testing technologies. Increased availability of non-invasive testing options is likely to enhance patient access to necessary healthcare services, thereby driving demand for Helicobacter Pylori testing. Furthermore, as healthcare providers invest in training and resources to implement these testing methods, the market is expected to experience robust growth. This trend reflects a broader commitment to improving healthcare delivery and outcomes, which is essential for the continued advancement of the Non-invasive Helicobacter Pylori Testing Market.
Rising Health Awareness and Preventive Care
The growing emphasis on health awareness and preventive care is significantly influencing the Non-invasive Helicobacter Pylori Testing Market. As individuals become more informed about the implications of Helicobacter Pylori infections, there is a marked increase in proactive health measures. This shift towards preventive healthcare is driving demand for non-invasive testing methods, as patients seek to identify potential health issues before they escalate. The market is witnessing a transformation, with healthcare providers increasingly recommending routine screenings, particularly in high-risk populations. This proactive approach not only aids in early diagnosis but also aligns with broader public health initiatives aimed at reducing the burden of gastrointestinal diseases, thereby fostering growth in the Non-invasive Helicobacter Pylori Testing Market.
Technological Advancements in Testing Methods
The Non-invasive Helicobacter Pylori Testing Market is experiencing a surge in technological advancements that enhance testing accuracy and efficiency. Innovations such as breath tests and stool antigen tests are becoming increasingly prevalent, providing reliable alternatives to invasive procedures like endoscopy. These non-invasive methods not only reduce patient discomfort but also lower the risk of complications associated with invasive testing. The market is projected to grow as these technologies become more widely adopted, with estimates suggesting a compound annual growth rate of over 8% in the coming years. As healthcare providers seek to improve patient outcomes, the integration of advanced testing technologies is likely to play a pivotal role in shaping the future of the Non-invasive Helicobacter Pylori Testing Market.
Increasing Incidence of Helicobacter Pylori Infections
The rising incidence of Helicobacter Pylori infections is a significant driver for the Non-invasive Helicobacter Pylori Testing Market. Studies indicate that nearly half of the world's population is infected with this bacterium, which is linked to various gastrointestinal disorders, including peptic ulcers and gastric cancer. As awareness of these health risks grows, there is a corresponding increase in demand for effective testing solutions. The market is responding to this need, with a projected increase in testing volumes, particularly in regions with high prevalence rates. This trend underscores the importance of accessible and non-invasive testing options, which are essential for early detection and management of infections, thereby propelling the Non-invasive Helicobacter Pylori Testing Market forward.