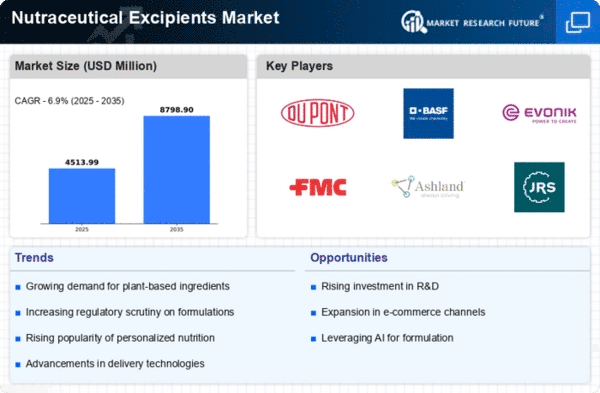
Market Trends
Key Emerging Trends in the Nutraceutical Excipients Market
In the nutraceutical market, key players find themselves obligated to adhere to government rules and regulations established to ensure compliance with industry standards. Regulatory authorities play a pivotal role in overseeing permits, formulating entry regulations for new participants, managing residual concerns, and scrutinizing expiration dates for specific products, particularly dietary supplements. In the United States, the Food and Drug Administration (FDA) takes on the responsibility of regulating the manufacturing and distribution of functional food and nutraceuticals.
The regulatory landscape involves stringent guidelines governing the introduction of various types of functional food, beverages, and nutraceuticals to the market. These regulations encompass the permissible quantity of ingredients, and criteria for labeling the diverse components that must be specified on product labels. Such regulatory oversight is essential for consumer safety, ensuring that products meet specified standards and adhere to accurate labeling practices.
The FDA, as a central regulatory authority in the U.S., plays a pivotal role in overseeing the nutraceutical market. It exercises control over the manufacturing processes and distribution channels of functional food and nutraceutical products. This control is crucial in upholding the integrity of the industry, ensuring that products reaching consumers are safe, effective, and accurately labeled.
One notable aspect of regulation involves the strict criteria dictating the types of functional food, beverages, and nutraceuticals that can be introduced to the market. This involves evaluating the safety and efficacy of these products before granting approval. The regulatory authorities also prescribe limits on the quantities of specific ingredients that can be used in these products. This serves to prevent the inclusion of excessive or potentially harmful substances that could compromise consumer well-being.
Labelling requirements form another critical aspect of regulation in the nutraceutical market. Guidelines set by regulatory authorities stipulate the information that must be included on product labels, ensuring transparency and providing consumers with essential details about the contents of the product. This includes accurate representation of ingredients, nutritional information, and other pertinent details that contribute to informed consumer choices.
However, one of the challenges arising from such stringent regulations is the potential restriction on market growth. The limited availability of excipient options in the market, influenced by regulatory constraints, can pose challenges for industry players. Excipients, which are inert substances often used as fillers or carriers in the production of pharmaceuticals and nutraceuticals, play a crucial role in product formulation. Regulatory limitations may constrain the choices available to manufacturers, impacting their ability to innovate and optimize product formulations.
Despite these challenges, the overarching goal of regulatory oversight is to safeguard consumer health and maintain the credibility of the nutraceutical market. By setting and enforcing standards, regulatory authorities seek to ensure that products in the market are of high quality, safe for consumption, and accurately represented to consumers. Industry players, in turn, must navigate these regulations with diligence, balancing the need for compliance with the imperative to foster innovation and growth in the dynamic nutraceutical landscape.


















Leave a Comment