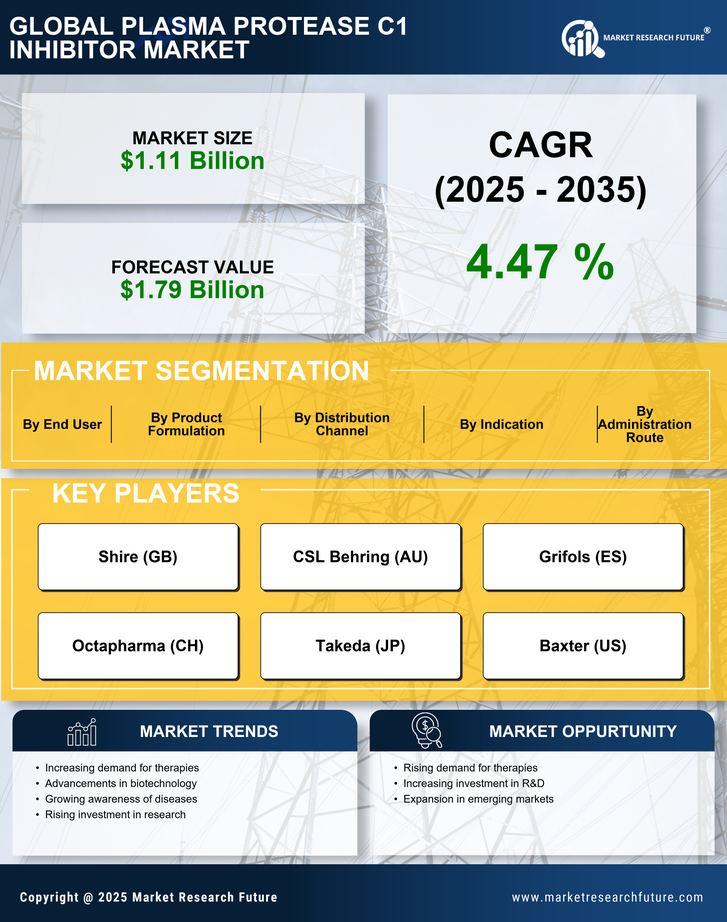
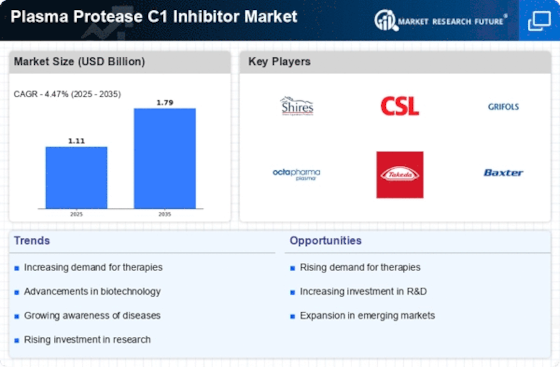
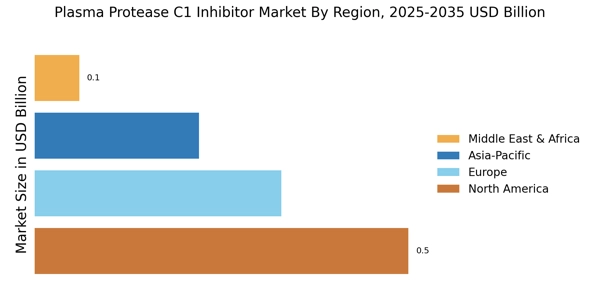
Rising Awareness and Education Initiatives
The increasing awareness and education initiatives surrounding hereditary angioedema are driving growth in the Plasma Protease C1 Inhibitor Market. Healthcare providers, patients, and advocacy groups are actively working to disseminate information about HAE, its symptoms, and available treatment options. This heightened awareness is leading to earlier diagnosis and treatment, which is crucial for managing the condition effectively. As more patients seek treatment, the demand for plasma protease C1 inhibitors is likely to rise. Additionally, educational programs aimed at healthcare professionals are improving the understanding of HAE, further contributing to the market's expansion as more practitioners become equipped to manage this rare disease.
Growing Prevalence of Hereditary Angioedema
The rising incidence of hereditary angioedema (HAE) is a primary driver for the Plasma Protease C1 Inhibitor Market. HAE is a genetic condition characterized by recurrent episodes of severe swelling, which can be life-threatening. As awareness of this condition increases, more patients are being diagnosed and treated. Recent estimates suggest that the prevalence of HAE is approximately 1 in 50,000 individuals, leading to a growing patient population in need of effective therapies. This surge in demand for plasma protease C1 inhibitors, which are critical in managing HAE, is likely to propel market growth. Furthermore, the increasing number of healthcare professionals specializing in rare diseases contributes to the identification and treatment of HAE, thereby expanding the market for plasma protease C1 inhibitors.
Regulatory Support for Innovative Therapies
Regulatory bodies are increasingly providing support for the development and approval of innovative therapies in the Plasma Protease C1 Inhibitor Market. Initiatives such as orphan drug designations and expedited review processes are encouraging pharmaceutical companies to invest in the development of new treatments for rare diseases like HAE. This regulatory environment fosters innovation and accelerates the availability of new therapies to patients in need. As a result, the market is likely to see a rise in the number of approved plasma protease C1 inhibitors, which will enhance treatment options for patients and drive overall market growth. The supportive regulatory landscape is a crucial factor in shaping the future of the plasma protease C1 inhibitor market.
Increasing Investment in Rare Disease Research
The growing investment in research and development for rare diseases is a significant driver for the Plasma Protease C1 Inhibitor Market. Governments and private organizations are increasingly allocating funds to support research initiatives aimed at understanding and treating rare conditions such as HAE. This trend is reflected in the rising number of clinical trials and studies focused on plasma protease C1 inhibitors. In recent years, the number of clinical trials for HAE treatments has increased, indicating a robust pipeline of potential therapies. This influx of investment not only fosters innovation but also enhances the likelihood of new product approvals, thereby expanding the market for plasma protease C1 inhibitors.
Technological Advancements in Drug Development
Technological innovations in drug development are significantly influencing the Plasma Protease C1 Inhibitor Market. Advances in biotechnology, such as recombinant DNA technology and monoclonal antibody production, have enhanced the efficacy and safety profiles of plasma protease C1 inhibitors. These innovations allow for the development of more targeted therapies, which are increasingly preferred by healthcare providers and patients alike. The market has witnessed a shift towards biologics, with a notable increase in the approval of new therapies that utilize these advanced technologies. As a result, the market is expected to expand, driven by the introduction of novel products that offer improved outcomes for patients suffering from conditions like HAE.