Regulatory Support and Incentives
Regulatory bodies are increasingly providing support and incentives for the development of therapies targeting Primary Biliary Cholangitis, which is a crucial driver for the Primary Biliary Cholangitis Therapeutic Market. Initiatives such as orphan drug designations and fast-track approvals are designed to encourage pharmaceutical companies to invest in treatments for rare diseases like PBC. These regulatory advantages can expedite the development process, allowing companies to bring new therapies to market more swiftly. Additionally, the potential for market exclusivity can enhance the financial viability of developing treatments for PBC, attracting more players to the market. This supportive regulatory environment is likely to foster innovation and competition, ultimately benefiting patients with PBC.
Integration of Personalized Medicine
The shift towards personalized medicine is emerging as a key driver in the Primary Biliary Cholangitis Therapeutic Market. Tailoring treatment approaches based on individual patient characteristics, such as genetic profiles and disease severity, is becoming increasingly feasible. This trend is supported by advancements in genomics and biomarker research, which enable healthcare providers to make more informed treatment decisions. Personalized medicine has the potential to enhance treatment efficacy and minimize adverse effects, thereby improving patient adherence to therapy. As the understanding of PBC pathophysiology deepens, the development of targeted therapies that align with personalized treatment strategies is likely to gain momentum. This evolution in treatment paradigms could significantly influence the landscape of the Primary Biliary Cholangitis Therapeutic Market.
Advancements in Research and Development
Innovations in research and development are pivotal in shaping the Primary Biliary Cholangitis Therapeutic Market. The emergence of novel therapies, including biologics and small molecules, has the potential to transform treatment paradigms for PBC. For instance, recent clinical trials have demonstrated the efficacy of new agents in improving liver function and patient outcomes. The investment in R&D by pharmaceutical companies is expected to yield a variety of treatment options, catering to diverse patient needs. Moreover, the collaboration between academic institutions and industry players is likely to enhance the pace of discovery and development of new therapies. As a result, the market may witness an influx of innovative products, which could significantly impact the therapeutic landscape for PBC.
Growing Awareness and Education Initiatives
The increasing awareness of Primary Biliary Cholangitis among healthcare professionals and the general public is a significant driver for the Primary Biliary Cholangitis Therapeutic Market. Educational initiatives aimed at improving understanding of PBC symptoms, diagnosis, and treatment options are gaining traction. As healthcare providers become more knowledgeable about the disease, they are better equipped to identify and manage PBC in patients. This heightened awareness is likely to lead to earlier diagnosis and treatment, which can improve patient outcomes. Furthermore, patient advocacy groups are playing a vital role in raising awareness and providing resources for those affected by PBC. As awareness continues to grow, the demand for effective therapies is expected to rise, thereby propelling the market forward.
Rising Prevalence of Primary Biliary Cholangitis
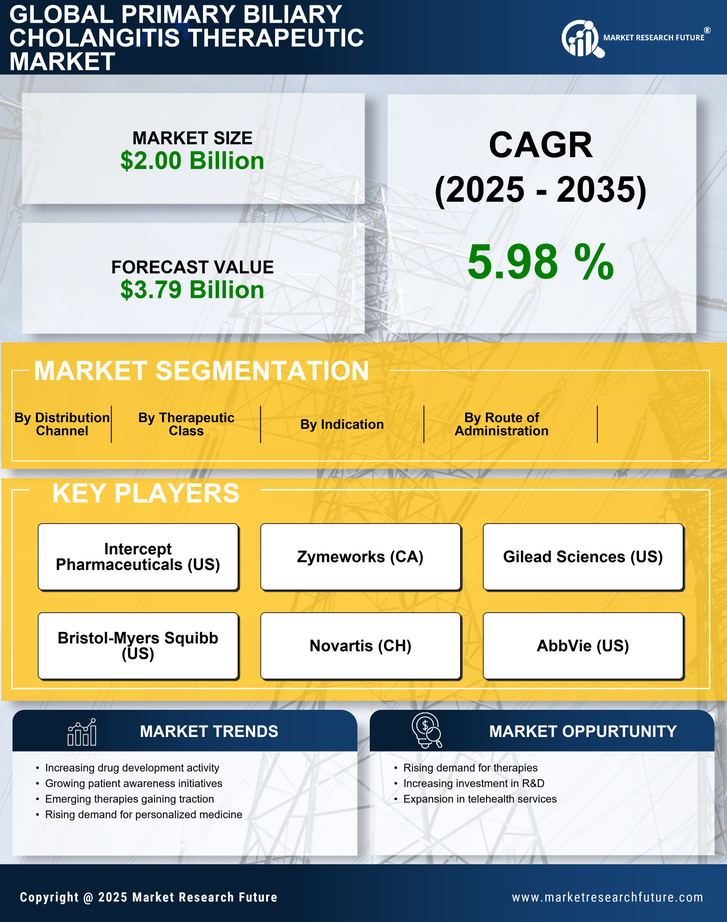
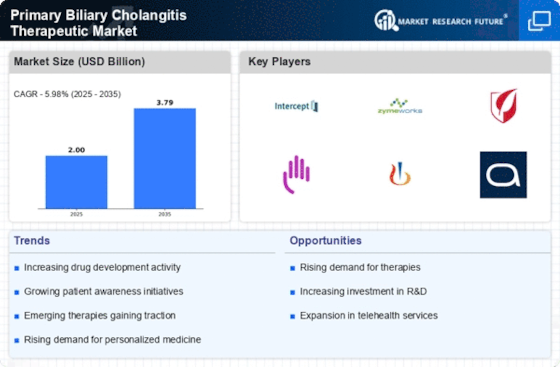
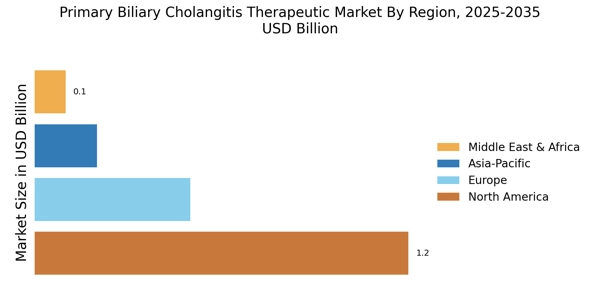
The increasing incidence of Primary Biliary Cholangitis (PBC) is a notable driver for the Primary Biliary Cholangitis Therapeutic Market. Recent estimates suggest that PBC affects approximately 1 in 1,000 women over the age of 40, indicating a growing patient population. This rise in prevalence necessitates the development and availability of effective therapeutic options. As awareness of the disease expands, more patients are likely to seek diagnosis and treatment, thereby propelling market growth. Furthermore, the aging population is expected to contribute to the increasing number of PBC cases, as the condition is predominantly diagnosed in middle-aged women. Consequently, pharmaceutical companies are likely to invest in research and development to address this unmet medical need, further stimulating the Primary Biliary Cholangitis Therapeutic Market.