Collaborative Research Efforts
Collaborative research initiatives among academic institutions, healthcare organizations, and industry stakeholders are emerging as a vital driver in the Primary Ciliary Dyskinesia Market. These partnerships aim to enhance understanding of PCD and accelerate the development of new therapies. By pooling resources and expertise, stakeholders can conduct more comprehensive studies, leading to improved diagnostic methods and treatment options. Such collaborations may also facilitate access to funding and grants, further propelling research efforts. The synergy created through these partnerships is likely to yield significant advancements in the management of PCD, ultimately benefiting patients and healthcare providers alike.
Advancements in Treatment Modalities
Innovations in treatment options for Primary Ciliary Dyskinesia Market are poised to transform the Primary Ciliary Dyskinesia Market. Recent developments in gene therapy and targeted therapies offer promising avenues for addressing the underlying genetic causes of PCD. These advancements may lead to more effective management strategies, potentially improving patient outcomes. The introduction of novel pharmacological agents and personalized medicine approaches could also enhance treatment efficacy. As the market evolves, the integration of these advanced therapies is likely to attract investment and research funding, further stimulating growth in the industry. The potential for improved quality of life for patients with PCD may drive demand for these innovative solutions.
Growing Awareness and Education Initiatives
The increasing awareness surrounding Primary Ciliary Dyskinesia Market is a critical driver in the Primary Ciliary Dyskinesia Market. Educational campaigns aimed at both healthcare providers and patients are essential for early diagnosis and intervention. As more information becomes available about the symptoms and implications of PCD, it is anticipated that healthcare professionals will be better equipped to recognize and diagnose the condition. This heightened awareness may lead to an increase in patient referrals for specialized care, thereby expanding the market. Additionally, patient advocacy groups are playing a pivotal role in disseminating information, which could further enhance the visibility of PCD and its associated challenges.
Regulatory Support for Research and Development
Regulatory bodies are increasingly supporting research and development initiatives in the Primary Ciliary Dyskinesia Market. This support may manifest in the form of expedited approval processes for new therapies and diagnostic tools. Such regulatory frameworks are designed to encourage innovation and facilitate the introduction of novel treatments to the market. The potential for orphan drug designation for therapies targeting PCD could also incentivize pharmaceutical companies to invest in this niche area. As a result, the regulatory environment is likely to foster a more dynamic market landscape, promoting the development of effective solutions for patients suffering from PCD.
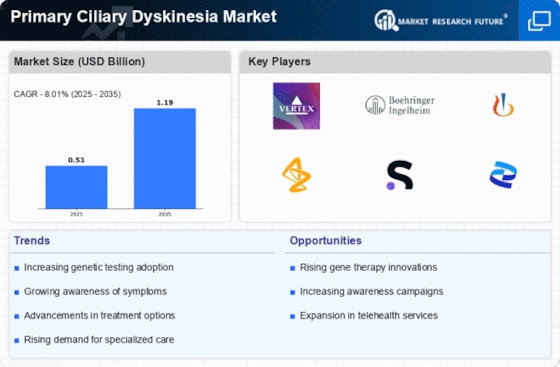
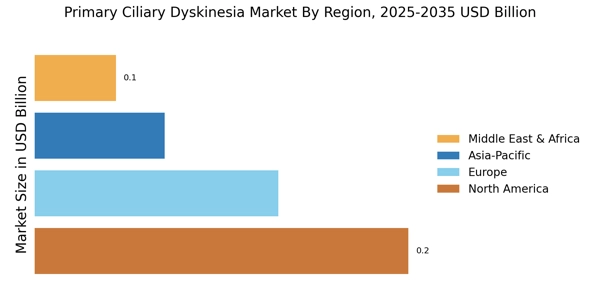
Increasing Prevalence of Primary Ciliary Dyskinesia
The rising incidence of Primary Ciliary Dyskinesia Market (PCD) is a notable driver in the Primary Ciliary Dyskinesia Market. Recent estimates suggest that PCD affects approximately 1 in 15,000 to 1 in 30,000 live births, indicating a growing patient population. This increase in prevalence is likely to elevate the demand for effective diagnostic and therapeutic options. As awareness of PCD expands among healthcare professionals and the general public, more individuals are being diagnosed, which could lead to a surge in market growth. Furthermore, the recognition of PCD as a significant contributor to respiratory diseases may prompt healthcare systems to allocate more resources towards research and treatment, thereby enhancing the overall market landscape.