Aging Population
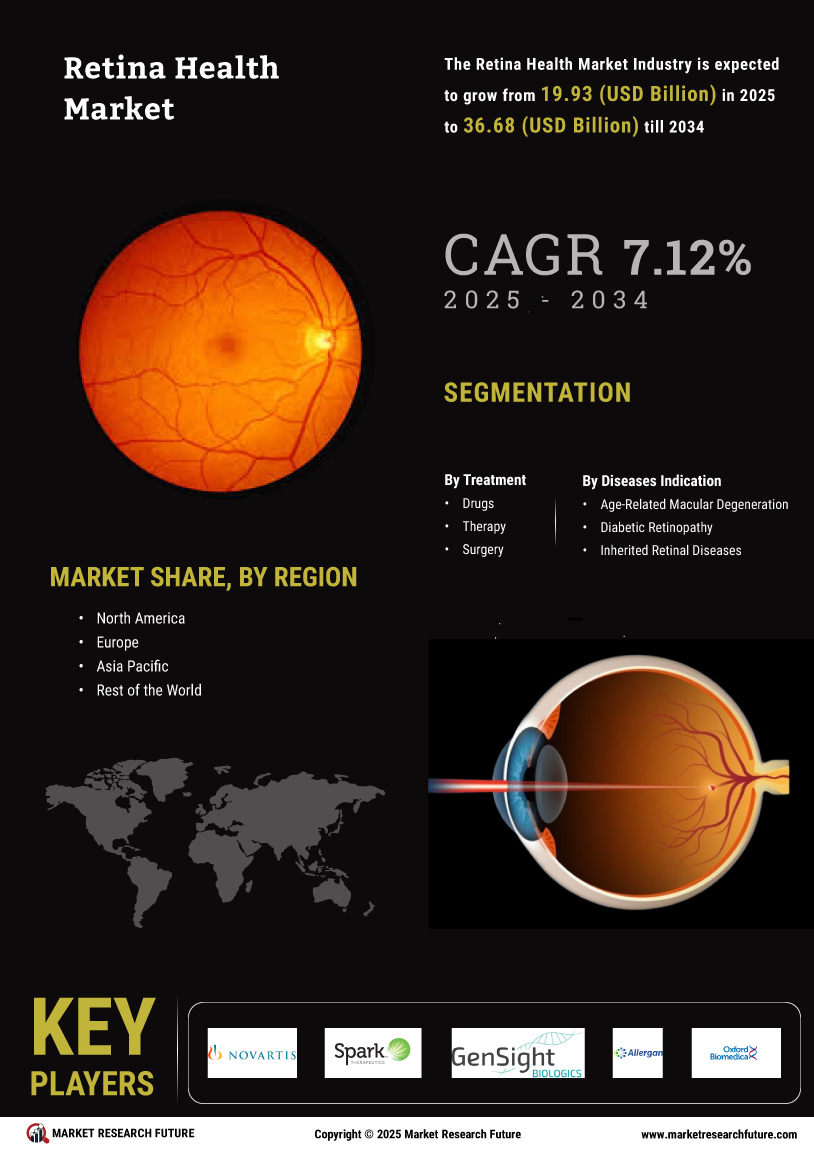
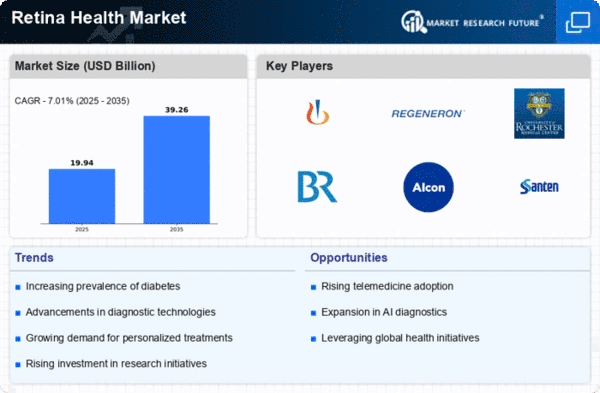
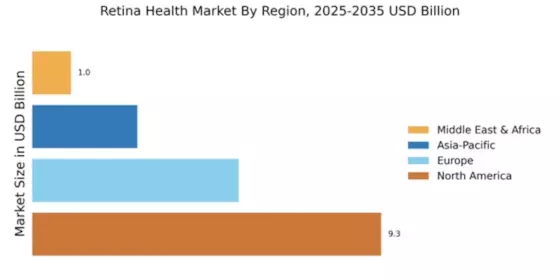
The aging population is a critical driver of the Global Retina Health Market Industry. As life expectancy increases, the incidence of age-related eye diseases, such as macular degeneration and cataracts, is also on the rise. This demographic shift necessitates enhanced retinal health services and treatments to address the specific needs of older adults. With a growing number of individuals requiring specialized eye care, the market is poised for substantial growth. Projections indicate that by 2035, the market could reach 39.3 USD Billion, underscoring the urgent need for effective interventions and support systems for the aging population.
Market Growth Projections
The Global Retina Health Market Industry is on a promising growth trajectory, with projections indicating a market value of 18.6 USD Billion in 2024 and an anticipated increase to 39.3 USD Billion by 2035. This growth is underpinned by a compound annual growth rate of 7.02% from 2025 to 2035. Such figures suggest a robust demand for retinal health solutions, driven by factors such as technological advancements, increased healthcare expenditure, and a rising prevalence of eye disorders. The market's expansion reflects a broader commitment to improving eye health outcomes globally.
Growing Awareness and Education
Awareness campaigns and educational initiatives regarding retinal health are gaining traction globally, significantly impacting the Global Retina Health Market Industry. Organizations are actively promoting the importance of regular eye examinations and early detection of retinal diseases. This heightened awareness is likely to lead to increased patient engagement and proactive healthcare-seeking behavior. As individuals become more informed about the risks associated with retinal disorders, the demand for preventive measures and treatments is expected to rise. Consequently, this trend may contribute to the market's growth trajectory, aligning with the overall increase in healthcare spending and technological advancements.
Increased Healthcare Expenditure
The Global Retina Health Market Industry is benefiting from rising healthcare expenditure across various regions. Governments and private sectors are investing more in healthcare infrastructure, which includes funding for eye care services. This increase in financial resources enables better access to retinal health treatments and technologies. For instance, countries with robust healthcare systems are likely to see a surge in the adoption of advanced retinal therapies. As a result, the market is expected to grow at a compound annual growth rate of 7.02% from 2025 to 2035, indicating a sustained commitment to improving eye health and addressing the needs of affected populations.
Rising Prevalence of Eye Disorders
The Global Retina Health Market Industry is experiencing growth driven by the increasing prevalence of eye disorders such as diabetic retinopathy and age-related macular degeneration. As populations age and lifestyle factors contribute to these conditions, the demand for retinal health solutions intensifies. In 2024, the market is projected to reach 18.6 USD Billion, reflecting a growing awareness and need for effective treatments. This trend is likely to continue, as the World Health Organization indicates that the number of individuals affected by visual impairments is expected to rise significantly in the coming years, further propelling the market.
Technological Advancements in Treatment
Technological innovations in the Global Retina Health Market Industry are transforming treatment modalities for retinal diseases. Advanced imaging techniques, such as optical coherence tomography, and minimally invasive surgical procedures enhance diagnostic accuracy and treatment efficacy. These advancements not only improve patient outcomes but also drive market growth as healthcare providers adopt new technologies. The integration of artificial intelligence in diagnostics and treatment planning is particularly noteworthy, as it appears to streamline processes and enhance precision. As these technologies continue to evolve, they are expected to contribute to the market's expansion, aligning with the projected growth to 39.3 USD Billion by 2035.