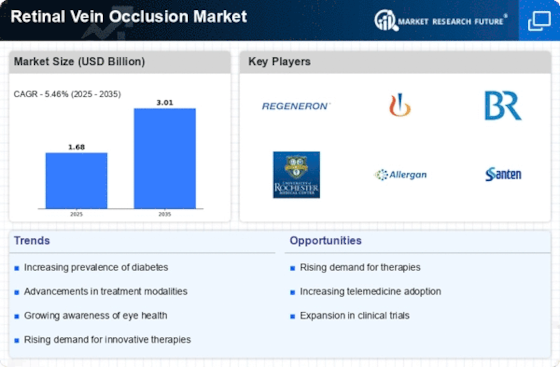
Top Industry Leaders in the Retinal Vein Occlusion Market
 Retinal Vein Occlusion Key Companies" width="600" height="300">*Disclaimer: List of key companies in no particular orderLatest Retinal Vein Occlusion Companies Update
Retinal Vein Occlusion Key Companies" width="600" height="300">*Disclaimer: List of key companies in no particular orderLatest Retinal Vein Occlusion Companies Update
-
October 2023: Roche submitted long-term evidence indicating a high probability of approval for the treatment of retinal vein occlusion (RVO) with Vabysmo. The data demonstrates that Vabysmo may effectively sustain visual improvements in patients with RVO, even with a dose interval extended to once every four months for some individuals. The outcomes are unsurprising given the prompt efficacy of Vabysmo, as demonstrated in prior studies that have indicated its capacity to decrease the frequency of dosage in comparison to the first formulation of Eylea by Regeneron and Bayer. Vabysmo, a pharmaceutical product, was granted approval in January 2022 for the treatment of wet age-related macular degeneration (AMD) and diabetic macular edema (DME). Since its approval, Vabysmo has demonstrated remarkable success, attaining blockbuster status with sales amounting to 957 million Swiss francs ($1.1 billion) for the initial half of the current year.
-
April 2023: The pharmaceutical companies STADA Arzneimittel and Xbrane Biopharma have jointly introduced Ximluci, a biosimilar of ranibizumab, across many European nations. This medication is intended for the therapeutic management of various ocular disorders. The Ximluci ranibizumab product is now accessible to ophthalmologists and their patients. The biosimilar, produced in Europe as a result of STADA's collaboration with Xbrane, aims to enhance patient accessibility to biological therapies and promote competition, so supporting the long-term viability of healthcare systems across Europe. According to the agreement, both entities bear the responsibility for the research and production of the biosimilars. STADA will possess the marketing authorization and commercial rights for the biosimilar in Europe and certain other markets.
List of Retinal Vein Occlusion Key companies in the market
- Allergan PLC
- Bayer
- Bristol-Myers Squibb
- Ellex Medical Lasers Ltd
- GlaxoSmithKline PLC
- IRIDEX Corporation
- Lumenis
- Novartis AG
- NIDEK CO., LTD
- Regeneron Pharmaceuticals
- Hoffmann-La Roche AG
- Quantel Medical Inc.
- Topcon Medical Systems, Inc.
- ZEISS