Rising Cardiovascular Disease Incidence
The increasing prevalence of cardiovascular diseases in South America is a crucial driver for the herg screening market. As healthcare providers focus on improving patient outcomes, the demand for effective screening methods has surged. Reports indicate that cardiovascular diseases account for approximately 30% of all deaths in the region, prompting regulatory bodies to emphasize cardiac safety in drug development. This heightened awareness has led to a growing need for herg screening solutions, as pharmaceutical companies seek to mitigate risks associated with drug-induced arrhythmias. Consequently, the herg screening market is likely to experience significant growth as stakeholders prioritize patient safety and regulatory compliance.
Investment in Biopharmaceutical Research
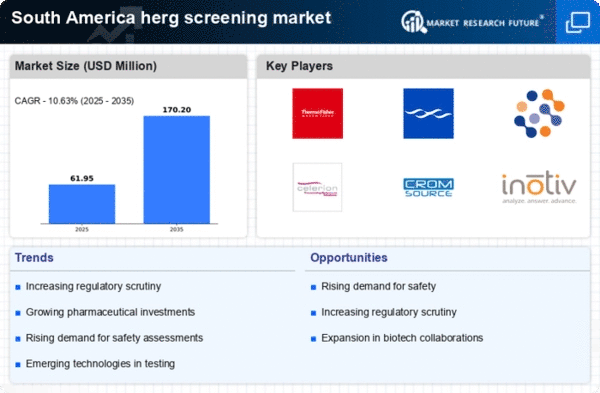
The herg screening market is witnessing a surge in investment from biopharmaceutical companies in South America. With the region's growing focus on innovative drug development, there is an increasing need for robust screening methods to ensure cardiac safety. In 2025, biopharmaceutical investments in South America are projected to reach $5 billion, reflecting a commitment to enhancing research capabilities. This influx of funding is likely to drive the adoption of advanced herg screening technologies, as companies aim to streamline their drug development processes while adhering to stringent regulatory requirements. As a result, the herg screening market stands to benefit from this trend, fostering innovation and improving patient safety.
Growing Awareness of Drug-Induced Arrhythmias
The herg screening market is experiencing growth due to the increasing awareness of drug-induced arrhythmias among healthcare professionals and regulatory bodies in South America. As the understanding of the risks associated with certain medications expands, there is a heightened emphasis on cardiac safety during drug development. This awareness is reflected in the rising number of training programs and workshops focused on herg screening methodologies. In 2025, it is anticipated that educational initiatives will increase by 15%, further promoting the importance of herg screening in ensuring patient safety. Consequently, the herg screening market is likely to benefit from this trend, as stakeholders prioritize effective screening practices.
Technological Integration in Drug Development
The herg screening market is being propelled by the integration of advanced technologies in drug development processes across South America. The adoption of high-throughput screening methods and automated systems is enhancing the efficiency and accuracy of herg screening. In 2025, it is projected that the market for automated screening technologies will grow by 25%, reflecting the industry's shift towards more sophisticated methodologies. This technological advancement not only streamlines the screening process but also reduces the time and costs associated with drug development. As a result, the herg screening market is likely to see increased investment and innovation, ultimately benefiting patient safety and therapeutic efficacy.
Regulatory Changes and Compliance Requirements
Recent regulatory changes in South America have significantly impacted the herg screening market. Authorities are increasingly mandating comprehensive cardiac safety assessments for new drug applications, which has heightened the demand for herg screening solutions. In 2025, it is estimated that compliance costs for pharmaceutical companies could rise by 20% due to these new regulations. This shift necessitates the integration of herg screening into the drug development pipeline, compelling companies to invest in advanced technologies and methodologies. As a result, the herg screening market is poised for growth, driven by the need for compliance and the assurance of patient safety in drug therapies.