Expansion of Clinical Trials
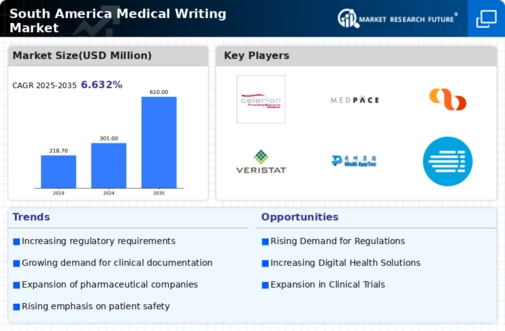
The medical writing market in South America is significantly influenced by the expansion of clinical trials across the region. Countries like Argentina and Brazil are becoming increasingly attractive for pharmaceutical companies seeking to conduct clinical research. The number of clinical trials has reportedly increased by 15% in the last year alone, creating a heightened demand for medical writing services. These services are essential for drafting protocols, informed consent forms, and clinical study reports, which are critical for regulatory submissions. As the clinical trial landscape evolves, the medical writing market is poised to grow, driven by the need for precise and compliant documentation.
Emergence of Biopharmaceutical Companies
The rise of biopharmaceutical companies in South America is reshaping the medical writing market. With a growing number of biotech firms entering the market, there is an increasing demand for specialized medical writing services tailored to the unique needs of these companies. Biopharmaceuticals often require extensive documentation for regulatory submissions, including detailed clinical trial data and product information. This trend is evident as the biopharmaceutical sector is projected to grow by 20% in the next five years. Consequently, the medical writing market must adapt to meet the specific requirements of these innovative companies, thereby driving growth and specialization within the industry.
Increased Focus on Patient-Centric Approaches
the medical writing market in South America is experiencing a shift towards patient-centric approaches in healthcare.. This trend emphasizes the importance of clear and accessible medical documentation that caters to patient needs. As healthcare providers strive to enhance patient engagement, the demand for medical writers who can create informative materials, such as patient leaflets and educational content, is on the rise. This focus on patient-centricity is likely to influence the medical writing market, as organizations seek to improve communication and understanding between healthcare professionals and patients. The potential for growth in this area suggests a promising future for medical writing services that prioritize patient needs.
Regulatory Changes and Compliance Requirements
The medical writing market in South America is significantly impacted by evolving regulatory changes and compliance requirements. As countries in the region enhance their regulatory frameworks, the demand for accurate and compliant medical writing becomes increasingly critical. For instance, new guidelines introduced by health authorities necessitate comprehensive documentation for drug approvals and clinical trials. This shift is likely to drive the medical writing market, as companies seek to ensure adherence to these regulations. The complexity of regulatory requirements may also lead to a greater reliance on professional medical writers, who possess the expertise to navigate these challenges effectively.
Rising Investment in Healthcare Infrastructure
The medical writing market in South America is experiencing a notable boost due to increased investment in healthcare infrastructure. Governments and private entities are allocating substantial funds to enhance healthcare facilities, which in turn drives the demand for high-quality medical documentation. For instance, Brazil has seen a surge in healthcare spending, with an estimated growth of 10% in 2025. This investment not only improves patient care but also necessitates comprehensive medical writing services to ensure compliance with regulatory standards. As healthcare systems expand, the need for skilled medical writers who can produce clear and concise documentation becomes paramount, thereby propelling the medical writing market forward.
















Leave a Comment