Growing Focus on Biosimilars
The increasing focus on biosimilars in South Korea is a notable driver for the host cell-protein testing market. As the biosimilar sector expands, the need for rigorous testing of host cell proteins becomes essential to ensure that these products are safe and effective. In 2025, the biosimilars market in South Korea is expected to grow by approximately 15%, reflecting a shift towards more affordable treatment options. This growth is accompanied by a demand for comprehensive testing to demonstrate similarity to reference biologics, which includes host cell-protein analysis. Consequently, the host cell-protein-testing market is likely to benefit from this trend, as manufacturers seek to validate the quality and safety of their biosimilar products through robust testing protocols.
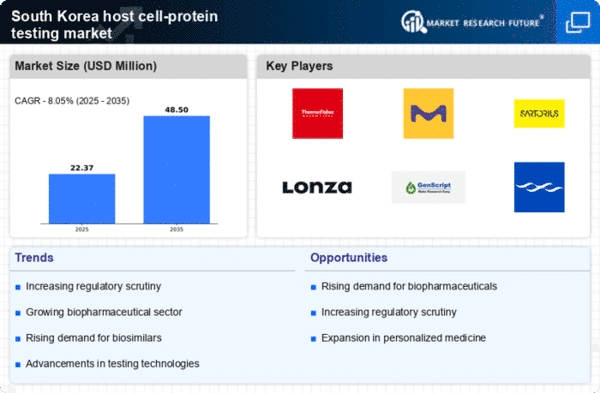
Increased Regulatory Scrutiny
The host cell-protein testing market is experiencing heightened regulatory scrutiny in South Korea, which serves as a crucial driver for its growth. Regulatory bodies are increasingly mandating comprehensive testing of host cell proteins to ensure the safety and quality of biopharmaceutical products. This trend is particularly evident in the context of the Korean Ministry of Food and Drug Safety (MFDS), which has implemented stringent guidelines for biopharmaceutical manufacturers. As a result, companies are compelled to invest in advanced testing methodologies to comply with these regulations. The MFDS has reported that non-compliance can lead to significant financial penalties, further incentivizing adherence to testing protocols. This regulatory environment not only fosters a culture of safety but also drives innovation within the host cell-protein-testing market, as firms seek to develop more efficient and reliable testing solutions.
Rising Biopharmaceutical Production
The increasing production of biopharmaceuticals in South Korea is a key driver for the host cell-protein testing market. As the biopharmaceutical sector expands, the demand for rigorous testing of host cell proteins becomes paramount to ensure product safety and efficacy. In 2025, the biopharmaceutical market in South Korea is projected to reach approximately $10 billion, reflecting a growth rate of around 8% annually. This growth necessitates enhanced testing protocols to comply with regulatory standards, thereby propelling the host cell-protein-testing market forward. Furthermore, the emphasis on quality assurance in biopharmaceutical manufacturing processes underscores the importance of host cell-protein testing, as it helps in identifying potential contaminants that could compromise product integrity. Consequently, the rising biopharmaceutical production is likely to significantly influence the host cell-protein-testing market in South Korea.
Advancements in Analytical Technologies
Advancements in analytical technologies are significantly influencing the host cell-protein testing market in South Korea. The development of more sophisticated testing methods, such as mass spectrometry and high-performance liquid chromatography (HPLC), enhances the accuracy and efficiency of host cell-protein detection. These technologies allow for the identification of low-abundance proteins and contaminants that may affect product quality. As the demand for high-quality biopharmaceuticals continues to rise, manufacturers are increasingly adopting these advanced analytical techniques to meet regulatory requirements and ensure product safety. The integration of automation and data analytics into testing processes further streamlines operations, potentially reducing time-to-market for new biopharmaceutical products. Thus, the ongoing advancements in analytical technologies are likely to propel the host cell-protein-testing market forward.
Emphasis on Quality Control in Manufacturing
The emphasis on quality control in biopharmaceutical manufacturing is a critical driver for the host cell-protein testing market. In South Korea, manufacturers are increasingly recognizing the importance of stringent quality control measures to ensure the safety and efficacy of their products. This focus is reflected in the adoption of Good Manufacturing Practices (GMP), which necessitate comprehensive testing of host cell proteins. As a result, companies are investing in robust testing frameworks to identify and mitigate potential risks associated with host cell proteins. The market for quality control solutions in biopharmaceuticals is projected to grow by approximately 10% annually, indicating a strong demand for host cell-protein testing services. This trend underscores the vital role of quality control in maintaining product integrity and compliance with regulatory standards, thereby driving the host cell-protein-testing market.