Rising Incidence of Cancer
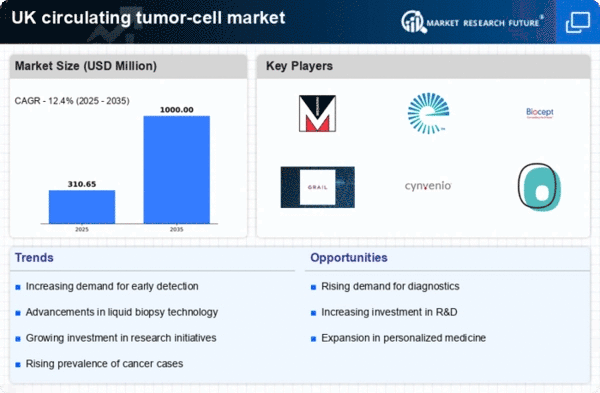
The increasing incidence of cancer in the UK is a primary driver for the circulating tumor-cell market. According to recent statistics, cancer cases are projected to rise by approximately 2.5% annually, leading to a heightened demand for innovative diagnostic solutions. This trend is likely to stimulate investment in research and development, as healthcare providers seek to implement advanced technologies for early detection and monitoring. The circulating tumor-cell market is positioned to benefit from this growing need, as these cells provide critical insights into tumor dynamics and treatment efficacy. As the population ages and lifestyle factors contribute to cancer prevalence, the market is expected to expand significantly, potentially reaching a valuation of £1 billion by 2027. This growth underscores the urgency for effective diagnostic tools in the fight against cancer.
Increased Investment in Cancer Research
The circulating tumor-cell market is significantly influenced by the rising investment in cancer research within the UK. Government and private sector funding for oncology research has seen a notable increase, with allocations reaching over £500 million in recent years. This financial support is aimed at fostering innovation in cancer diagnostics and therapeutics, including the development of circulating tumor-cell technologies. As researchers explore the complexities of cancer biology, the demand for advanced diagnostic tools is expected to rise, propelling the market forward. Furthermore, partnerships between academic institutions and industry players are likely to enhance the development of novel applications for circulating tumor cells, thereby expanding their utility in clinical settings and driving market growth.
Advancements in Liquid Biopsy Technologies
Technological innovations in liquid biopsy are transforming the landscape of the circulating tumor-cell market. These advancements enable non-invasive sampling of blood to detect circulating tumor cells, offering a less invasive alternative to traditional biopsy methods. The UK market is witnessing a surge in the adoption of these technologies, with a projected growth rate of 15% annually. This shift is driven by the increasing recognition of the benefits of liquid biopsies, including real-time monitoring of treatment responses and the ability to detect minimal residual disease. As healthcare providers and researchers continue to explore the potential of liquid biopsies, the circulating tumor-cell market is likely to experience substantial growth, fostering collaborations between technology developers and clinical institutions to enhance diagnostic capabilities.
Growing Awareness of Early Cancer Detection
There is a growing awareness among healthcare professionals and patients regarding the importance of early cancer detection, which is a significant driver for the circulating tumor-cell market. Educational campaigns and initiatives aimed at promoting screening and early diagnosis are gaining traction in the UK. This heightened awareness is likely to lead to increased demand for innovative diagnostic solutions, including those that utilize circulating tumor cells. As patients become more informed about their health, the market is expected to see a rise in the adoption of these technologies. The potential for early intervention and improved patient outcomes is driving healthcare providers to invest in circulating tumor-cell diagnostics, thereby contributing to the overall growth of the market.
Regulatory Support for Innovative Diagnostics
Regulatory bodies in the UK are increasingly supportive of innovative diagnostic solutions, which is positively impacting the circulating tumor-cell market. Streamlined approval processes and initiatives aimed at fostering innovation are encouraging the development and commercialization of new technologies. This regulatory environment is conducive to the introduction of advanced circulating tumor-cell assays, which can provide critical information for personalized treatment strategies. As regulatory frameworks evolve to accommodate these innovations, the market is likely to experience accelerated growth. The collaboration between regulatory agencies and industry stakeholders is essential for ensuring that new diagnostic tools meet safety and efficacy standards, ultimately enhancing patient care and driving market expansion.