Regulatory Framework Enhancements
The regulatory framework surrounding the theranostics market in the UK is evolving to support innovation and ensure patient safety. Recent updates to regulatory guidelines are streamlining the approval process for theranostic products, making it easier for companies to bring new solutions to market. This regulatory support is crucial for fostering a conducive environment for innovation, as it encourages investment in the development of novel therapies and diagnostics. As a result, the theranostics market is expected to see an influx of new products, enhancing treatment options for patients. The improved regulatory landscape is likely to stimulate market growth, as companies are more inclined to invest in the development of theranostic solutions.
Advancements in Biomarker Discovery
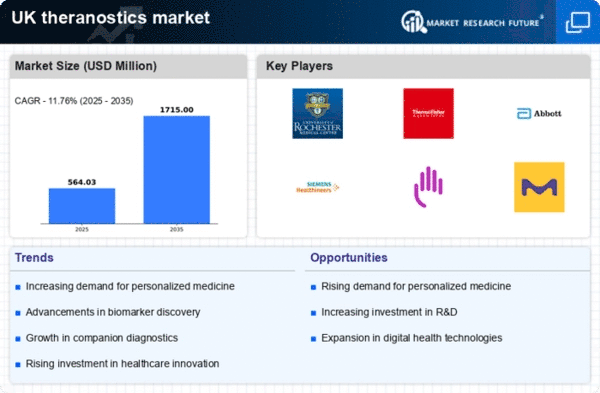
The theranostics market in the UK is experiencing a surge due to advancements in biomarker discovery. These innovations enable the identification of specific biological markers that can predict patient responses to therapies. As a result, the market is projected to grow at a CAGR of approximately 12% over the next five years. This growth is driven by the increasing need for personalized medicine, which allows for tailored treatment plans based on individual patient profiles. The integration of advanced technologies, such as next-generation sequencing and proteomics, is enhancing the accuracy of biomarker identification. Consequently, the theranostics market is likely to expand as healthcare providers seek to implement more effective and targeted treatment strategies, ultimately improving patient outcomes.
Growing Awareness of Personalized Medicine
There is a notable increase in awareness regarding personalized medicine among healthcare professionals and patients in the UK, which is positively impacting the theranostics market. As patients become more informed about their treatment options, the demand for tailored therapies is rising. This shift in patient expectations is prompting healthcare providers to adopt theranostic approaches that align with individual patient needs. In 2025, it is estimated that over 60% of oncologists in the UK will incorporate theranostic strategies into their practice. This growing acceptance of personalized medicine is likely to drive the expansion of the theranostics market, as more healthcare providers seek to implement targeted therapies that enhance treatment efficacy.
Collaboration Between Academia and Industry
The collaboration between academic institutions and industry players is fostering innovation within the theranostics market in the UK. These partnerships facilitate the exchange of knowledge and resources, leading to the development of cutting-edge diagnostic and therapeutic solutions. In recent years, several collaborative initiatives have emerged, focusing on the integration of research findings into clinical practice. This synergy is expected to accelerate the translation of scientific discoveries into viable theranostic applications. As a result, the theranostics market is poised for growth, as these collaborations enhance the speed and efficiency of product development, ultimately benefiting patients through improved treatment options.
Increased Investment in Research and Development
Investment in research and development (R&D) is a critical driver for the theranostics market in the UK. With the growing emphasis on precision medicine, pharmaceutical companies and research institutions are allocating substantial resources to develop novel theranostic products. In 2025, R&D spending in the UK healthcare sector is expected to reach £5 billion, reflecting a commitment to innovation. This influx of funding supports the exploration of new therapeutic targets and the development of companion diagnostics, which are essential for the successful implementation of theranostics. As a result, the theranostics market is likely to benefit from a robust pipeline of new products, enhancing treatment options for patients and driving market growth.